Abstract
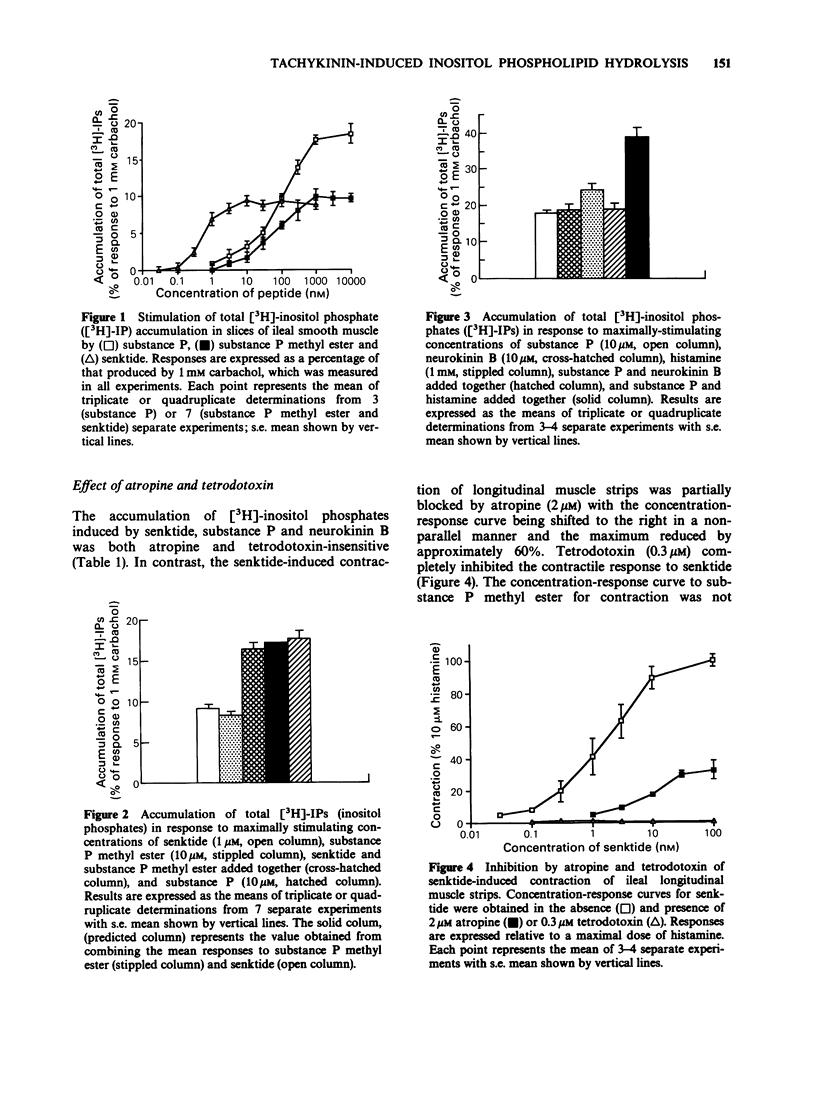
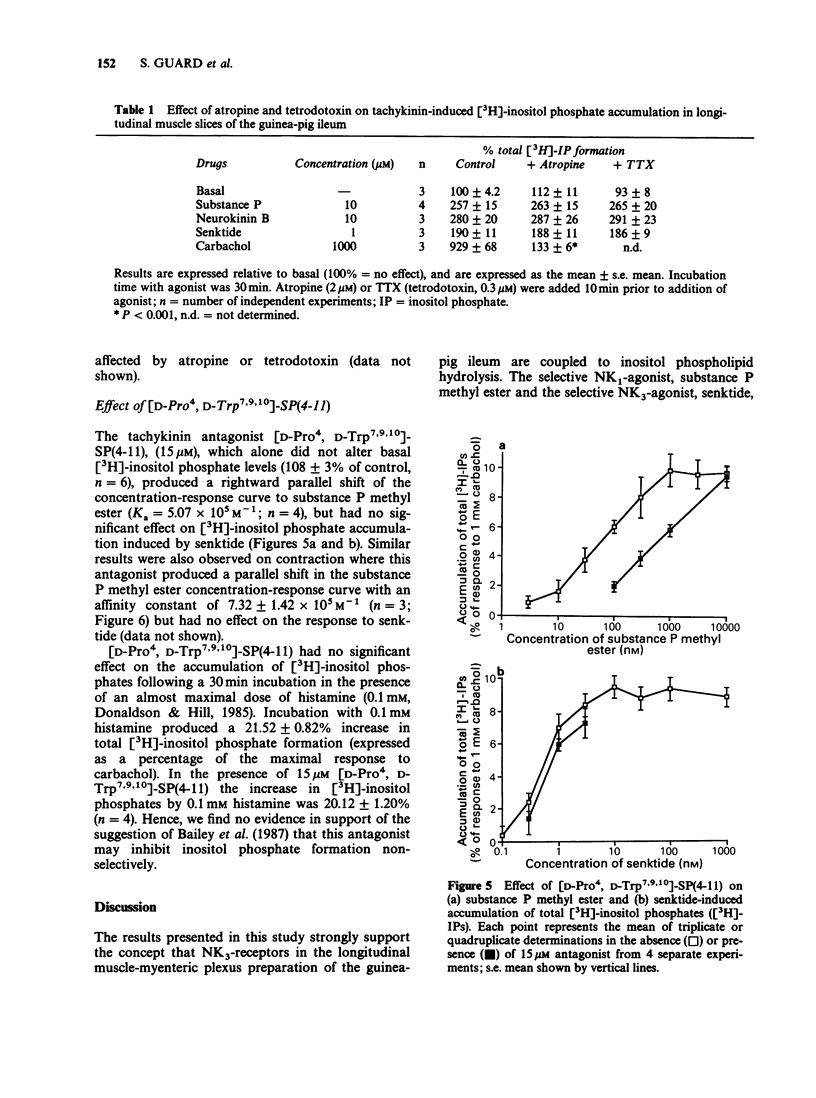
1. Tachykinin-stimulated inositol phospholipid hydrolysis was examined in slices of longitudinal muscle from guinea-pig ileum. 2. Substance P, neurokinin A and neurokinin B induced a concentration-dependent accumulation of total [3H]-inositol phosphates in the presence of 12 mM lithium with similar maximal responses and EC50 values. 3. The selective NK1-receptor agonist, substance P methyl ester, and the selective NK3-receptor agonist succ-[Asp6, MePhe8]-SP(6-11) (senktide) also stimulated [3H]-inositol phosphate formation with maximum responses of 50.69 +/- 0.96 and 45.64 +/- 1.17% relative to 10 microM substance P, respectively. Substance P methyl ester was approximately equipotent with substance P, whereas senktide was approximately 100 times more potent. 4. When added together, maximally effective concentrations of substance P methyl ester and senktide gave responses that were fully additive. In contrast, responses to substance P and neurokinin B were not additive. 5. The stimulation of [3H]-inositol phosphate formation by substance P, neurokinin B and senktide was not affected by atropine (2 microM) or tetrodotoxin (TTX, 0.3 microM). 6. The contractile effect of senktide was inhibited completely by TTX and partially blocked by atropine. Contractions induced by substance P methyl ester were not changed in the presence of TTX or atropine. 7. [D-Pro4, D-Trp7,9,10]-SP(4-11) competitively antagonized the action of substance P methyl ester on inositol phospholipid hydrolysis and contraction, but had no significant effect on senktide-induced inositol phospholipid breakdown or contraction. 8. These results suggest that NK3-receptors in the guinea-pig ileum are coupled to inositol phospholipid hydrolysis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey S. J., Lippe I. T., Holzer P. Effect of the tachykinin antagonist, [D-Pro4, D-Trp7,9,10] substance P-(4-11), on tachykinin- and histamine-induced inositol phosphate generation in intestinal smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1987 Mar;335(3):296–300. doi: 10.1007/BF00172800. [DOI] [PubMed] [Google Scholar]
- Bergström L., Torrens Y., Saffroy M., Beaujouan J. C., Lavielle S., Chassaing G., Morgat J. L., Glowinski J., Marquet A. [3H]neurokinin B and 125I-Bolton Hunter eledoisin label identical tachykinin binding sites in the rat brain. J Neurochem. 1987 Jan;48(1):125–133. doi: 10.1111/j.1471-4159.1987.tb13136.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow D. R., Curtis N. R., Suman-Chauhan N., Watling K. J., Williams B. J. Effects of tachykinins on inositol phospholipid hydrolysis in slices of hamster urinary bladder. Br J Pharmacol. 1987 Jan;90(1):211–217. doi: 10.1111/j.1476-5381.1987.tb16842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J., Hill S. J. Histamine-induced inositol phospholipid breakdown in the longitudinal smooth muscle of guinea-pig ileum. Br J Pharmacol. 1985 Jun;85(2):499–512. doi: 10.1111/j.1476-5381.1985.tb08887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusting G. J., Li D. M. Catecholamine release and potentiation of thromboxane A2 production by nicotine in the greyhound. Br J Pharmacol. 1986 Jan;87(1):29–36. doi: 10.1111/j.1476-5381.1986.tb10153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone R. L., Fosbraey P., Morton I. K. A comparison of the effects of three substance P antagonists on tachykinin-stimulated [3H]-acetylcholine release in the guinea-pig ileum. Br J Pharmacol. 1986 Jan;87(1):73–77. doi: 10.1111/j.1476-5381.1986.tb10158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosbraey P., Featherstone R. L., Morton I. K. Comparison of potency of substance P and related peptides on [3H]-acetylcholine release, and contractile actions, in the guinea-pig ileum. Naunyn Schmiedebergs Arch Pharmacol. 1984 Jun;326(2):111–115. doi: 10.1007/BF00517306. [DOI] [PubMed] [Google Scholar]
- Franco R., Costa M., Furness J. B. Evidence for the release of endogenous substance P from intestinal nerves. Naunyn Schmiedebergs Arch Pharmacol. 1979 Apr;306(3):195–201. doi: 10.1007/BF00507103. [DOI] [PubMed] [Google Scholar]
- Hanley M. R., Lee C. M., Michell R. H., Jones L. M. Similar effects of substance P and related peptides on salivation and on phosphatidylinositol turnover in rat salivary glands. Mol Pharmacol. 1980 Jul;18(1):78–83. [PubMed] [Google Scholar]
- Holzer P., Lembeck F. Neurally mediated contraction of ileal longitudinal muscle by substance P. Neurosci Lett. 1980 Apr;17(1-2):101–105. doi: 10.1016/0304-3940(80)90069-5. [DOI] [PubMed] [Google Scholar]
- Laufer R., Wormser U., Friedman Z. Y., Gilon C., Chorev M., Selinger Z. Neurokinin B is a preferred agonist for a neuronal substance P receptor and its action is antagonized by enkephalin. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7444–7448. doi: 10.1073/pnas.82.21.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. M., Campbell N. J., Williams B. J., Iversen L. L. Multiple tachykinin binding sites in peripheral tissues and in brain. Eur J Pharmacol. 1986 Nov 4;130(3):209–217. doi: 10.1016/0014-2999(86)90270-0. [DOI] [PubMed] [Google Scholar]
- Lee C. M., Iversen L. L., Hanley M. R., Sandberg B. E. The possible existence of multiple receptors for substance P. Naunyn Schmiedebergs Arch Pharmacol. 1982 Mar;318(4):281–287. doi: 10.1007/BF00501166. [DOI] [PubMed] [Google Scholar]
- Osakada F., Kubo K., Goto K., Kanazawa I., Munekata E. The contractile activities of neurokinin A, B and related peptides on smooth muscles. Eur J Pharmacol. 1986 Jan 21;120(2):201–208. doi: 10.1016/0014-2999(86)90541-8. [DOI] [PubMed] [Google Scholar]
- RANG H. P. STIMULANT ACTIONS OF VOLATILE ANAESTHETICS ON SMOOTH MUSCLE. Br J Pharmacol Chemother. 1964 Apr;22:356–365. doi: 10.1111/j.1476-5381.1964.tb02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoli D., Drapeau G., Dion S., D'Orléans-Juste P. Pharmacological receptors for substance P and neurokinins. Life Sci. 1987 Jan 12;40(2):109–117. doi: 10.1016/0024-3205(87)90349-3. [DOI] [PubMed] [Google Scholar]
- Watson S. P., Downes C. P. Substance P induced hydrolysis of inositol phospholipids in guinea-pig ileum and rat hypothalamus. Eur J Pharmacol. 1983 Sep 30;93(3-4):245–253. doi: 10.1016/0014-2999(83)90144-9. [DOI] [PubMed] [Google Scholar]
- Watson S. P., Sandberg B. E., Hanley M. R., Iversen L. L. Tissue selectivity of substance P alkyl esters: suggesting multiple receptors. Eur J Pharmacol. 1983 Jan 28;87(1):77–84. doi: 10.1016/0014-2999(83)90052-3. [DOI] [PubMed] [Google Scholar]
- Watson S. P. The action of substance P on contraction, inositol phospholipids and adenylate cyclase in rat small intestine. Biochem Pharmacol. 1984 Dec 1;33(23):3733–3737. doi: 10.1016/0006-2952(84)90033-9. [DOI] [PubMed] [Google Scholar]
- Wormser U., Laufer R., Hart Y., Chorev M., Gilon C., Selinger Z. Highly selective agonists for substance P receptor subtypes. EMBO J. 1986 Nov;5(11):2805–2808. doi: 10.1002/j.1460-2075.1986.tb04571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau W. M., Youther M. L. Direct evidence for a release of acetylcholine from the myenteric plexus of guinea pig small intestine by substance P. Eur J Pharmacol. 1982 Jul 30;81(4):665–668. doi: 10.1016/0014-2999(82)90357-0. [DOI] [PubMed] [Google Scholar]


