Abstract
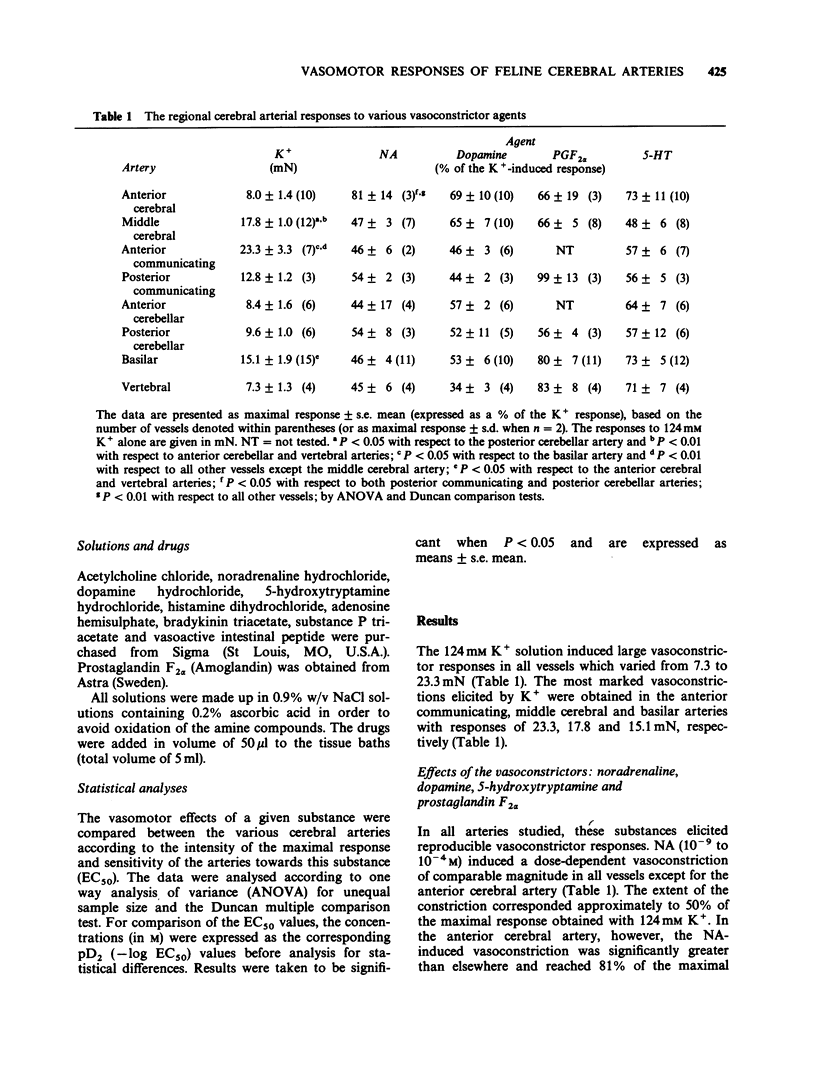
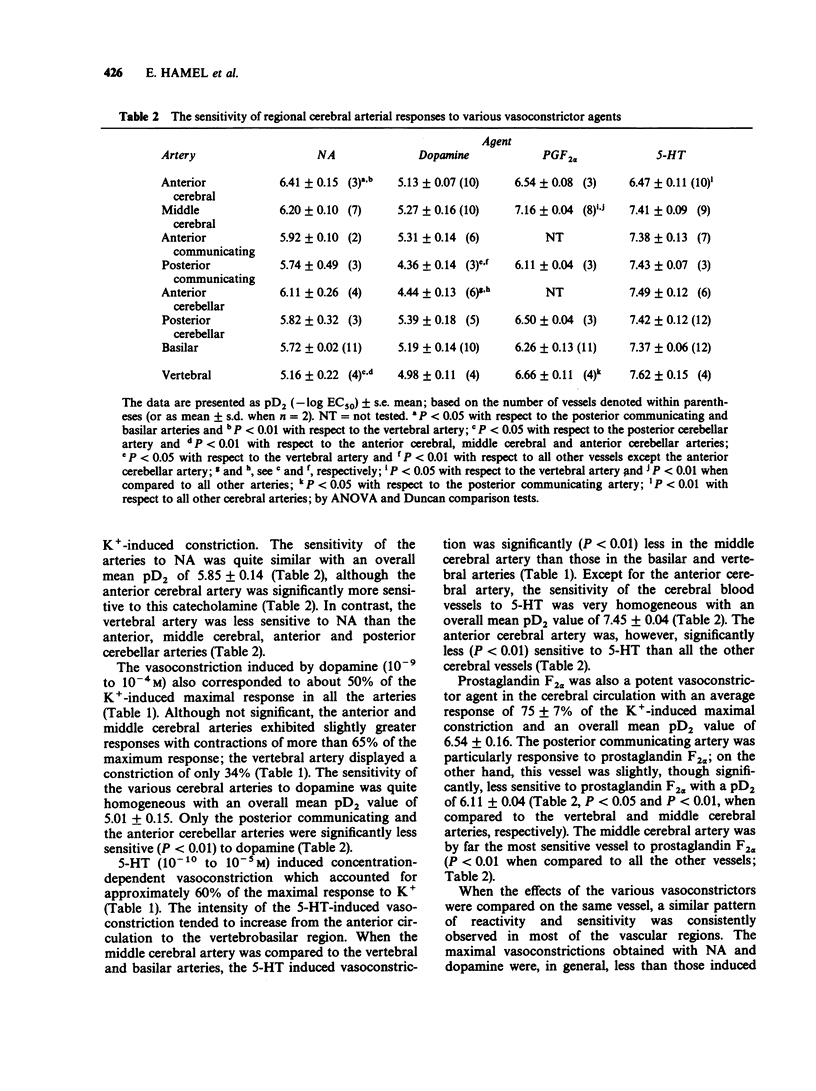
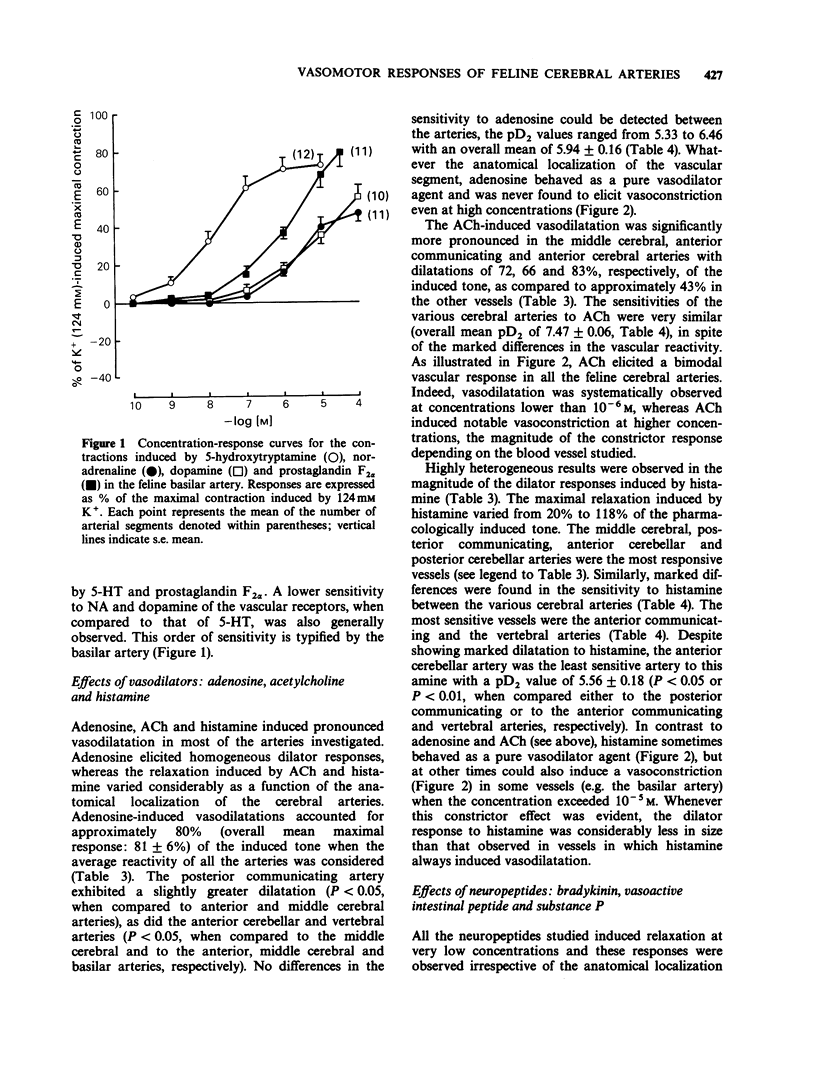
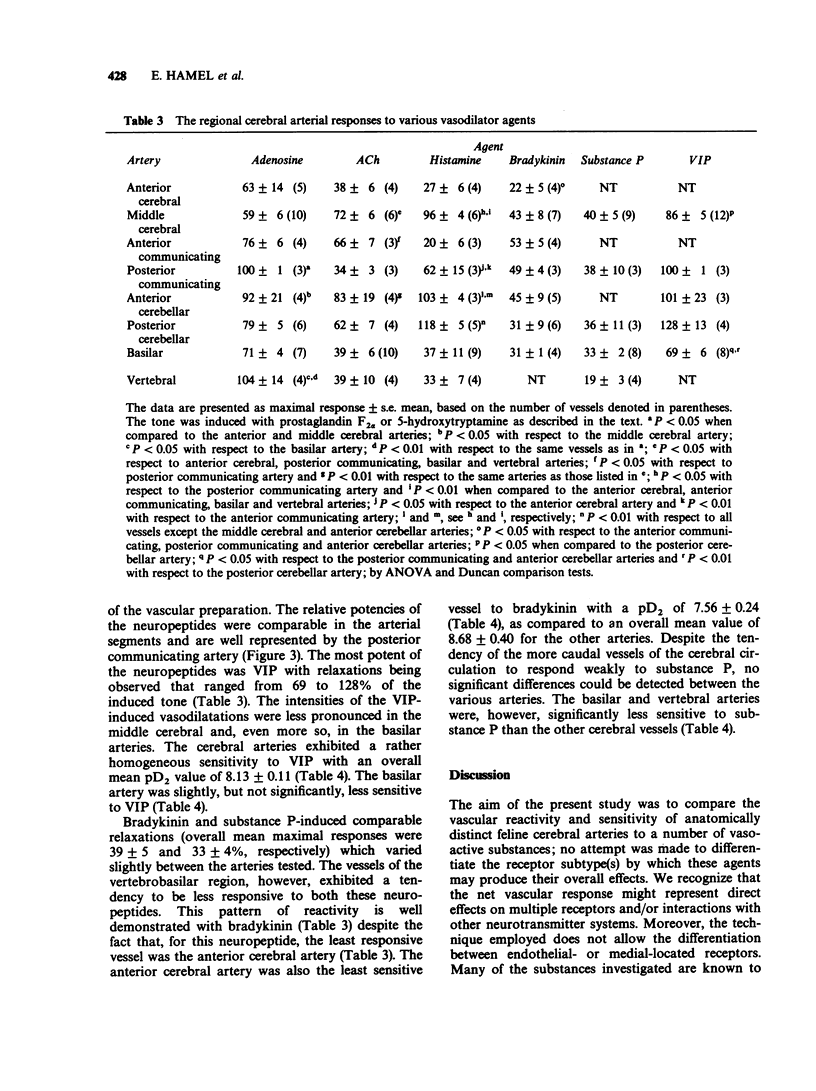
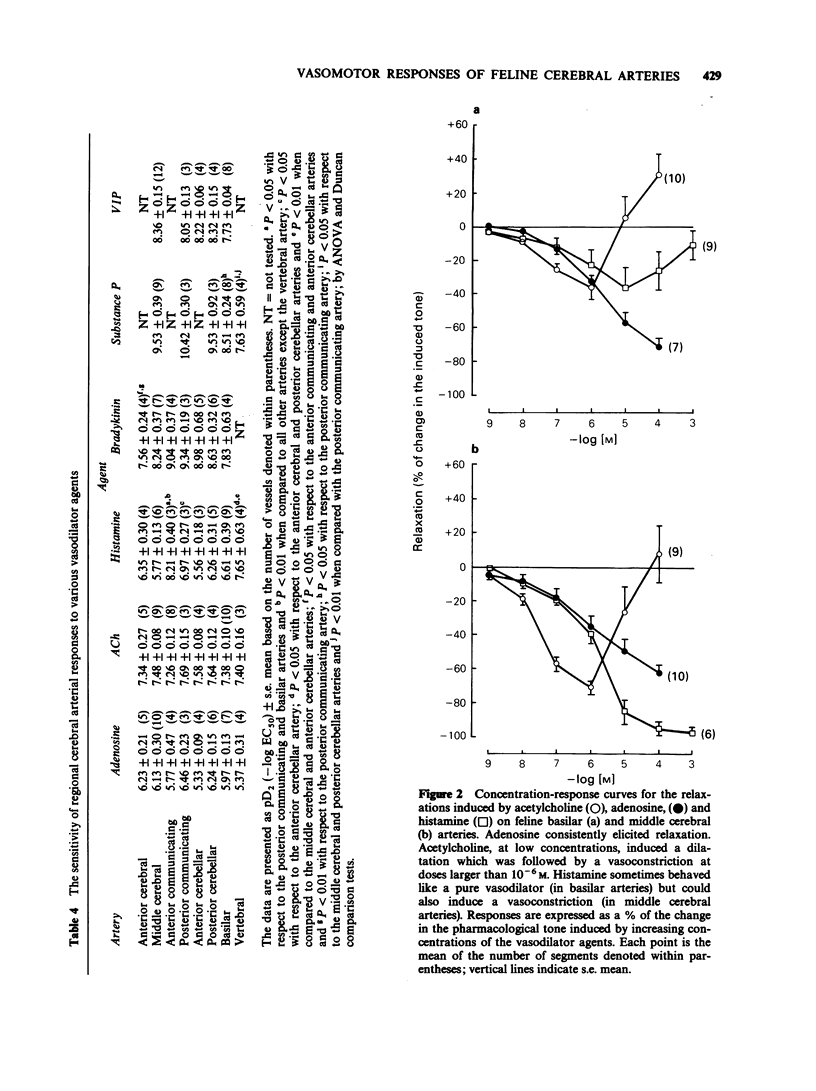
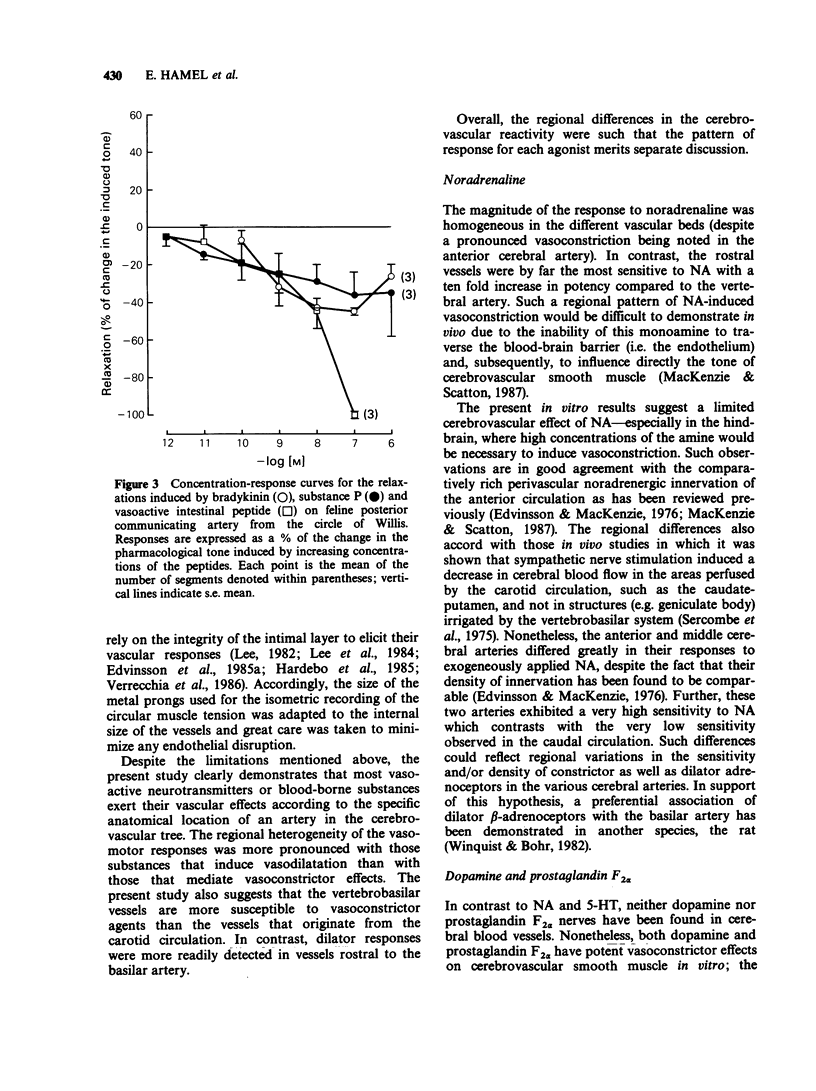
1. The vasomotor reactivity to a number of neurotransmitters and blood-borne substances was evaluated in several anatomically distinct arteries of the cat cerebral circulation. Few regional differences were observed in their vasoconstrictor responses to noradrenaline, dopamine, 5-hydroxytryptamine and prostaglandin F2 alpha. Only the anterior cerebral artery reacted strongly to all vasoconstrictor agents. 2. Adenosine, acetylcholine and histamine induced pronounced relaxation in the vast majority of the major cerebral arteries. The relaxation elicited by adenosine showed a slight degree of heterogeneity between the arteries and the overall response accounted for 81 +/- 6% of the pharmacologically-induced tone. On the other hand, the dilatation induced by acetylcholine and histamine varied as a function of the anatomical localization of the cerebral arteries. The acetylcholine-induced vasodilatation was significantly more pronounced in the middle cerebral, anterior communicating and anterior cerebellar arteries, with respective responses of 72, 66 and 83% of the induced tone as compared to 43% in the other vessels. However, all arteries were equally sensitive to acetylcholine with an overall mean pD2 value of 7.47 +/- 0.06. The most heterogeneous results were obtained with histamine and applied both to the magnitude of the maximal response and the sensitivity of the various arteries to this amine. The intensity of the relaxation varied from 20% (anterior communicating artery) to 118% (posterior cerebellar artery). 3. Among the neuropeptides studied, substance P and bradykinin were considerably less potent than vasoactive intestinal peptide on all the cerebral arteries. The least responsive vessel to bradykinin was the anterior cerebral artery with a maximal response of 22 +/- 5% of the induced-tone and a pD2 value of 7.56 +/- 0.24. All vessels responded weakly to substance P and those from the vertebrobasilar circulation were significantly less sensitive to this neuropeptide with pD2 values around 8.07 as compared to 9.82 in the more rostral arteries. Although all vessels were equally sensitive to vasoactive intestinal peptide, the dilator responses were significantly less pronounced in the middle cerebral and basilar arteries (maximal response of 86 +/- 5% and 69 +/- 6% of the induced-tone, respectively, as compared to 110 +/- 9% in the other vessels). 4. The vertebrobasilar arteries were as reactive, if not more reactive, to vasoconstrictors than the vessels originating from the carotid circulation. In contrast, the dilator responses were less marked in most caudal arteries.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan J. A., Buga G. M., Florence V. M., Gonsalves A., Snowden A. Distribution of choline acetyltransferase in cerebral and extracerebral cranial arteries of the cat. Its relationship to neurogenic atropine-sensitive dilation. Circ Res. 1982 Apr;50(4):470–476. doi: 10.1161/01.res.50.4.470. [DOI] [PubMed] [Google Scholar]
- Bradley P. B., Humphrey P. P., Williams R. H. Evidence for the existence of 5-hydroxytryptamine receptors, which are not of the 5-HT2 type, mediating contraction of rabbit isolated basilar artery. Br J Pharmacol. 1986 Jan;87(1):3–4. doi: 10.1111/j.1476-5381.1986.tb10149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J. E., Bevan J. A. Evidence that vasoactive intestinal polypeptide (VIP) mediates neurogenic vasodilation of feline cerebral arteries. Stroke. 1986 Nov-Dec;17(6):1189–1192. doi: 10.1161/01.str.17.6.1189. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. Serotonin axons in the supra- and subependymal plexuses and in the leptomeninges; their roles in local alterations of cerebrospinal fluid and vasomotor activity. Brain Res. 1976 Jan 30;102(1):103–130. doi: 10.1016/0006-8993(76)90578-3. [DOI] [PubMed] [Google Scholar]
- Chiba S., Tsuji T. Vascular responsiveness of isolated, perfused basilar arteries in dogs and monkeys. Tohoku J Exp Med. 1985 Jul;146(3):363–370. doi: 10.1620/tjem.146.363. [DOI] [PubMed] [Google Scholar]
- De Ley G., Weyne J., Demeester G., Leusen I. Response of local blood flow in the caudate nucleus of the cat to intraventricular administration of histamine. Stroke. 1982 Jul-Aug;13(4):499–504. doi: 10.1161/01.str.13.4.499. [DOI] [PubMed] [Google Scholar]
- Duckles S. P., Buck S. H. Substance P in the cerebral vasculature: depletion by capsaicin suggests a sensory role. Brain Res. 1982 Aug 5;245(1):171–174. doi: 10.1016/0006-8993(82)90355-9. [DOI] [PubMed] [Google Scholar]
- Duckles S. P. Evidence for a functional cholinergic innervation of cerebral arteries. J Pharmacol Exp Ther. 1981 Jun;217(3):544–548. [PubMed] [Google Scholar]
- Duckles S. P., Said S. I. Vasoactive intestinal peptide as a neurotransmitter in the cerebral circulation. Eur J Pharmacol. 1982 Mar 12;78(3):371–374. doi: 10.1016/0014-2999(82)90041-3. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Birath E., Uddman R., Lee T. J., Duverger D., MacKenzie E. T., Scatton B. Indoleaminergic mechanisms in brain vessels; localization, concentration, uptake and in vitro responses of 5-hydroxytryptamine. Acta Physiol Scand. 1984 Jul;121(3):291–299. doi: 10.1111/j.1748-1716.1984.tb07459.x. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Degueurce A., Duverger D., MacKenzie E. T., Scatton B. Central serotonergic nerves project to the pial vessels of the brain. Nature. 1983 Nov 3;306(5938):55–57. doi: 10.1038/306055a0. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Fahrenkrug J., Hanko J., Owman C., Sundler F., Uddman R. VIP (vasoactive intestinal polypeptide)-containing nerves of intracranial arteries in mammals. Cell Tissue Res. 1980;208(1):135–142. doi: 10.1007/BF00234179. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Falck B., Owman C. Possibilities for a cholinergic action on smooth musculature and on sympathetic axons in brain vessels mediated by muscarinic and nicotinic receptors. J Pharmacol Exp Ther. 1977 Jan;200(1):117–126. [PubMed] [Google Scholar]
- Edvinsson L., Fredholm B. B. Characterization of adenosine receptors in isolated cerebral arteries of cat. Br J Pharmacol. 1983 Dec;80(4):631–637. doi: 10.1111/j.1476-5381.1983.tb10052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L., Fredholm B. B., Hamel E., Jansen I., Verrecchia C. Perivascular peptides relax cerebral arteries concomitant with stimulation of cyclic adenosine monophosphate accumulation or release of an endothelium-derived relaxing factor in the cat. Neurosci Lett. 1985 Jul 31;58(2):213–217. doi: 10.1016/0304-3940(85)90166-1. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Gross P. M., Mohamed A. Characterization of histamine receptors in cat cerebral arteries in vitro and in situ. J Pharmacol Exp Ther. 1983 Apr;225(1):168–175. [PubMed] [Google Scholar]
- Edvinsson L., Hardebo J. E., McCulloch J., Owman C. Effects of dopaminergic agonists and antagonists on isolated cerebral blood vessels. Acta Physiol Scand. 1978 Nov;104(3):349–359. doi: 10.1111/j.1748-1716.1978.tb06286.x. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Hardebo J. E., Owman C. Pharmacological analysis of 5-hydroxytryptamine receptors in isolated intracranial and extracranial vessels of cat and man. Circ Res. 1978 Jan;42(1):143–151. doi: 10.1161/01.res.42.1.143. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., MacKenzie E. T. Amine mechanisms in the cerebral circulation. Pharmacol Rev. 1976 Dec;28(4):275–348. [PubMed] [Google Scholar]
- Edvinsson L., McCulloch J., Sharkey J. Vasomotor responses of cerebral arterioles in situ to putative dopamine receptor agonists. Br J Pharmacol. 1985 Jun;85(2):403–410. doi: 10.1111/j.1476-5381.1985.tb08875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L., McCulloch J., Uddman R. Substance P: immunohistochemical localization and effect upon cat pial arteries in vitro and in situ. J Physiol. 1981 Sep;318:251–258. doi: 10.1113/jphysiol.1981.sp013862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L., Nielsen K. C., Owman C., Sporrong B. Cholinergic mechanisms in pial vessels. Histochemistry, electron microscopy and pharmacology. Z Zellforsch Mikrosk Anat. 1972;134(3):311–325. doi: 10.1007/BF00307168. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Owman C. A pharmacologic comparison of histamine receptors in isolated extracranial and intracranial arteries in vitro. Neurology. 1975 Mar;25(3):271–276. doi: 10.1212/wnl.25.3.271. [DOI] [PubMed] [Google Scholar]
- Faraci F. M., Heistad D. D., Mayhan W. G. Role of large arteries in regulation of blood flow to brain stem in cats. J Physiol. 1987 Jun;387:115–123. doi: 10.1113/jphysiol.1987.sp016566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence V. M., Bevan J. A. Biochemical determinations of cholinergic innervation in cerebral arteries. Circ Res. 1979 Aug;45(2):212–218. doi: 10.1161/01.res.45.2.212. [DOI] [PubMed] [Google Scholar]
- Forrester T., Harper A. M., MacKenzie E. T., Thomson E. M. Effect of adenosine triphosphate and some derivatives on cerebral blood flow and metabolism. J Physiol. 1979 Nov;296:343–355. doi: 10.1113/jphysiol.1979.sp013009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gibbins I. L., Brayden J. E., Bevan J. A. Perivascular nerves with immunoreactivity to vasoactive intestinal polypeptide in cephalic arteries of the cat: distribution, possible origins and functional implications. Neuroscience. 1984 Dec;13(4):1327–1346. doi: 10.1016/0306-4522(84)90301-4. [DOI] [PubMed] [Google Scholar]
- Gross P. M., Harper A. M., Teasdale G. M. Cerebral circulation and histamine: 1. Participation of vascular H1- and H2-receptors in vasodilatory responses to carotid arterial infusion. J Cereb Blood Flow Metab. 1981;1(1):97–108. doi: 10.1038/jcbfm.1981.10. [DOI] [PubMed] [Google Scholar]
- Hamel E., Assumel-Lurdin C., Edvinsson L., MacKenzie E. T. Cholinergic innervation of small pial vessels: specific uptake and release processes. Acta Physiol Scand Suppl. 1986;552:13–16. [PubMed] [Google Scholar]
- Harder D. R., Gradall K., Madden J. A., Kampine J. P. Cellular actions of halothane on cat cerebral arterial muscle. Stroke. 1985 Jul-Aug;16(4):680–683. doi: 10.1161/01.str.16.4.680. [DOI] [PubMed] [Google Scholar]
- Harper A. M., MacKenzie E. T. Effects of 5-hydroxytryptamine on pial arteriolar calibre in anaesthetized cats. J Physiol. 1977 Oct;271(3):735–746. doi: 10.1113/jphysiol.1977.sp012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Rorstad O. P. Cerebral vascular adenylate cyclase: evidence for coupling to receptors for vasoactive intestinal peptide and parathyroid hormone. J Neurochem. 1984 Sep;43(3):849–856. doi: 10.1111/j.1471-4159.1984.tb12808.x. [DOI] [PubMed] [Google Scholar]
- Huang M., Rorstad O. P. Effects of vasoactive intestinal polypeptide, monoamines, prostaglandins, and 2-chloroadenosine on adenylate cyclase in rat cerebral microvessels. J Neurochem. 1983 Mar;40(3):719–726. doi: 10.1111/j.1471-4159.1983.tb08038.x. [DOI] [PubMed] [Google Scholar]
- Högestätt E. D., Andersson K. E., Edvinsson L. Mechanical properties of rat cerebral arteries as studied by a sensitive device for recording of mechanical activity in isolated small blood vessels. Acta Physiol Scand. 1983 Jan;117(1):49–61. doi: 10.1111/j.1748-1716.1983.tb07178.x. [DOI] [PubMed] [Google Scholar]
- Kapadia S. E., de Lanerolle N. C. Immunohistochemical and electron microscopic demonstration of vascular innervation in the mammalian brainstem. Brain Res. 1984 Jan 30;292(1):33–39. doi: 10.1016/0006-8993(84)90887-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Kyoshima K., Olschowka J. A., Jacobowitz D. M. Vasoactive intestinal polypeptide immunoreactive and cholinergic nerves in the whole mount preparation of the major cerebral arteries of the rat. Histochemistry. 1983;79(3):377–381. doi: 10.1007/BF00491773. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Tsukahara S., Sugita K., Nagata T. Adrenergic and cholinergic innervation of rat cerebral arteries. Consecutive demonstration on whole mount preparations. Histochemistry. 1981;70(2):129–138. doi: 10.1007/BF00493205. [DOI] [PubMed] [Google Scholar]
- Lee T. J. Cholinergic mechanism in the large cat cerebral artery. Circ Res. 1982 Jun;50(6):870–879. doi: 10.1161/01.res.50.6.870. [DOI] [PubMed] [Google Scholar]
- Lee T. J., Saito A., Berezin I. Vasoactive intestinal polypeptide-like substance: the potential transmitter for cerebral vasodilation. Science. 1984 May 25;224(4651):898–901. doi: 10.1126/science.6719122. [DOI] [PubMed] [Google Scholar]
- Liu-Chen L. Y., Mayberg M. R., Moskowitz M. A. Immunohistochemical evidence for a substance P-containing trigeminovascular pathway to pial arteries in cats. Brain Res. 1983 May 23;268(1):162–166. doi: 10.1016/0006-8993(83)90402-x. [DOI] [PubMed] [Google Scholar]
- MacKenzie E. T., Scatton B. Cerebral circulatory and metabolic effects of perivascular neurotransmitters. CRC Crit Rev Clin Neurobiol. 1987;2(4):357–419. [PubMed] [Google Scholar]
- Marco E. J., Balfagón G., Salaices M., Sánchez-Ferrer C. F., Marín J. Serotonergic innervation of cat cerebral arteries. Brain Res. 1985 Jul 8;338(1):137–139. doi: 10.1016/0006-8993(85)90255-0. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Shiosaka S., Matsumoto M., Yoneda S., Kimura K., Abe H., Hayakawa T., Inoue H., Tohyama M. Overall distribution of vasoactive intestinal polypeptide-containing nerves on the wall of cerebral arteries: an immunohistochemical study using whole-mounts. Neuroscience. 1983 Sep;10(1):89–96. doi: 10.1016/0306-4522(83)90083-0. [DOI] [PubMed] [Google Scholar]
- McCulloch J., Edvinsson L. Cerebral circulatory and metabolic effects of vasoactive intestinal polypeptide. Am J Physiol. 1980 Apr;238(4):H449–H456. doi: 10.1152/ajpheart.1980.238.4.H449. [DOI] [PubMed] [Google Scholar]
- Medgett I. C., Langer S. Z. Characterisation of smooth muscle alpha-adrenoceptors and of responses to electrical stimulation in the cat isolated perfused middle cerebral artery. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jun;323(1):24–32. doi: 10.1007/BF00498823. [DOI] [PubMed] [Google Scholar]
- Poulin P., Suzuki Y., Lederis K., Rorstad O. P. Autoradiographic localization of binding sites for vasoactive intestinal peptide (VIP) in bovine cerebral arteries. Brain Res. 1986 Sep 3;381(2):382–384. doi: 10.1016/0006-8993(86)90094-6. [DOI] [PubMed] [Google Scholar]
- Reinhard J. F., Jr, Liebmann J. E., Schlosberg A. J., Moskowitz M. A. Serotonin neurons project to small blood vessels in the brain. Science. 1979 Oct 5;206(4414):85–87. doi: 10.1126/science.482930. [DOI] [PubMed] [Google Scholar]
- Saito A., Lee T. J. Serotonin as an alternative transmitter in sympathetic nerves of large cerebral arteries of the rabbit. Circ Res. 1987 Feb;60(2):220–228. doi: 10.1161/01.res.60.2.220. [DOI] [PubMed] [Google Scholar]
- Saito A., Wu J. Y., Lee T. J. Evidence for the presence of cholinergic nerves in cerebral arteries: an immunohistochemical demonstration of choline acetyltransferase. J Cereb Blood Flow Metab. 1985 Jun;5(2):327–334. doi: 10.1038/jcbfm.1985.42. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Kassell N. F., Torner J. C., Maixner W., Turner D. M. Pharmacological comparison of isolated monkey and dog cerebral arteries. Stroke. 1985 May-Jun;16(3):482–489. doi: 10.1161/01.str.16.3.482. [DOI] [PubMed] [Google Scholar]
- Scatton B., Duverger D., L'Heureux R., Serrano A., Fage D., Nowicki J. P., MacKenzie E. T. Neurochemical studies on the existence, origin and characteristics of the serotonergic innervation of small pial vessels. Brain Res. 1985 Oct 21;345(2):219–229. doi: 10.1016/0006-8993(85)90997-7. [DOI] [PubMed] [Google Scholar]
- Sercombe R., Aubineau P., Edvinsson L., Mamo H., Owman C. H., Pinard E., Seylaz J. Neurogenic influence on local cerebral blood flow. Effect of catecholamines or sympathetic stimulation as correlated with the sympathetic innervation. Neurology. 1975 Oct;25(10):954–963. doi: 10.1212/wnl.25.10.954. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., McMaster D., Huang M., Lederis K., Rorstad O. P. Characterization of functional receptors for vasoactive intestinal peptide in bovine cerebral arteries. J Neurochem. 1985 Sep;45(3):890–899. doi: 10.1111/j.1471-4159.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Toda N. Actions of bradykinin on isolated cerebral and peripheral arteries. Am J Physiol. 1977 Mar;232(3):H267–H274. doi: 10.1152/ajpheart.1977.232.3.H267. [DOI] [PubMed] [Google Scholar]
- Toda N., Okamura T., Miyazaki M. Heterogeneity in the response to vasoconstrictors of isolated dog proximal and distal middle cerebral arteries. Eur J Pharmacol. 1984 Nov 13;106(2):291–299. doi: 10.1016/0014-2999(84)90716-7. [DOI] [PubMed] [Google Scholar]
- Toda N. Regional differences in the response to nicotine in isolated canine arteries. Eur J Pharmacol. 1976 Jan;35(1):151–160. doi: 10.1016/0014-2999(76)90310-1. [DOI] [PubMed] [Google Scholar]
- Uski T. K., Andersson K. E. Effects of prostanoids on isolated feline cerebral arteries. I. Characterization of the contraction-mediating receptor. Acta Physiol Scand. 1984 Jan;120(1):131–136. doi: 10.1111/j.1748-1716.1984.tb07382.x. [DOI] [PubMed] [Google Scholar]
- Uski T. K., Edvinsson L., Owman C. Effects of prostaglandin E1, E2 and E2 alpha on isolated pial arteries of cat. Acta Physiol Scand. 1981 Apr;111(4):487–490. doi: 10.1111/j.1748-1716.1981.tb06767.x. [DOI] [PubMed] [Google Scholar]
- Usui H., Fujiwara M., Tsukahara T., Taniguchi T., Kurahashi K. Differences in contractile responses to electrical stimulation and alpha-adrenergic binding sites in isolated cerebral arteries of humans, cows, dogs, and monkeys. J Cardiovasc Pharmacol. 1985;7 (Suppl 3):S47–S52. doi: 10.1097/00005344-198500073-00006. [DOI] [PubMed] [Google Scholar]
- Verbeuren T. J., Jordaens F. H., Herman A. G. Accumulation and release of [3H]-5-hydroxytryptamine in saphenous veins and cerebral arteries of the dog. J Pharmacol Exp Ther. 1983 Aug;226(2):579–588. [PubMed] [Google Scholar]
- Verrecchia C., Hamel E., Edvinsson L., MacKenzie E. T., Seylaz J. Role of the endothelium in the pial artery responses to several vasoactive peptides. Acta Physiol Scand Suppl. 1986;552:33–36. [PubMed] [Google Scholar]
- Wahl M., Kuschinsky W. The dilatatory action of adenosine on pial arteries of cats and its inhibition by theophylline. Pflugers Arch. 1976 Mar 11;362(1):55–59. doi: 10.1007/BF00588681. [DOI] [PubMed] [Google Scholar]
- Wahl M., Kuschinsky W. The dilating effect of histamine on pial arteries of cats and its mediation by H2 receptors. Circ Res. 1979 Feb;44(2):161–165. doi: 10.1161/01.res.44.2.161. [DOI] [PubMed] [Google Scholar]
- Wahl M., Young A. R., Edvinsson L., Wagner F. Effects of bradykinin on pial arteries and arterioles in vitro and in situ. J Cereb Blood Flow Metab. 1983 Jun;3(2):231–237. doi: 10.1038/jcbfm.1983.31. [DOI] [PubMed] [Google Scholar]
- Whalley E. T., Wahl M. Analysis of bradykinin receptor mediating relaxation of cat cerebral arteries in vivo and in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jun;323(1):66–71. doi: 10.1007/BF00498830. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., O'Neill J. T., Said S. I., Traystman R. J. Vasoactive intestinal polypeptide and the canine cerebral circulation. Circ Res. 1981 Jan;48(1):138–148. doi: 10.1161/01.res.48.1.138. [DOI] [PubMed] [Google Scholar]
- Winquist R. J., Bohr D. F. Characterization of the rat basilar artery in vitro. Experientia. 1982 Oct 15;38(10):1187–1188. doi: 10.1007/BF01959732. [DOI] [PubMed] [Google Scholar]


