Abstract
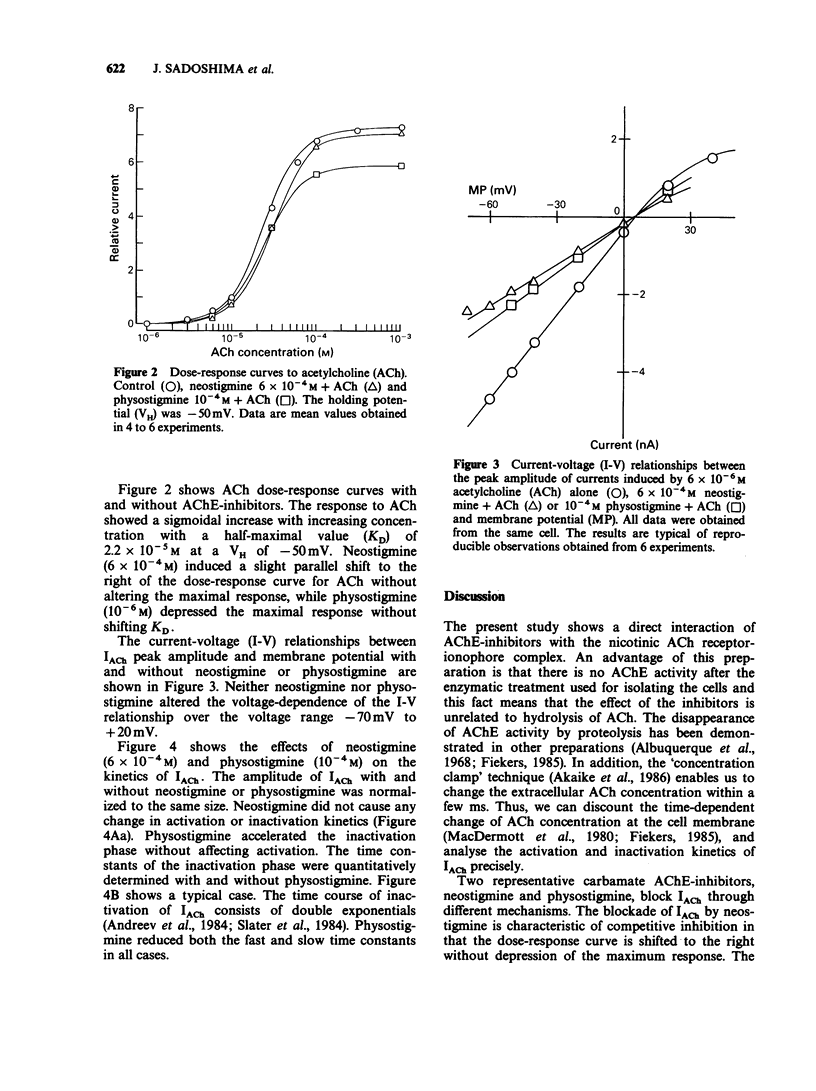
1. The effects of neostigmine and physostigmine, reversible carbamate acetylcholinesterase (AChE)-inhibitors, on nicotinic acetylcholine-induced inward currents (IACh) were investigated in enzymatically isolated single sympathetic ganglion cells from the bullfrog. The 'concentration clamp' technique which combines intracellular perfusion with a rapid external solution change under single electrode voltage-clamp conditions was used. 2. Pretreatment with neostigmine and physostigmine did not enhance IACh at any concentrations, suggesting that AChE activity had already disappeared during the enzymatic treatment of the preparation. 3. Both neostigmine and physostigmine inhibited IACh in a dose-dependent manner with IC50 values of 7.0 x 10(-4) M and 1.1 x 10(-4) M, respectively. The blockade by neostigmine was competitive, while that by physostigmine was non-competitive. 4. The inhibition of IACh by neostigmine and physostigmine showed no apparent voltage dependency. 5. Neostigmine did not cause obvious changes of the kinetics of IACh. However, physostigmine reduced both the fast and slow time constants of inactivation of IACh, thus facilitating the rate of inactivation without affecting the activation kinetics of IACh. 6. These results suggest that neostigmine and physostigmine have different direct actions on the ACh receptor-ionophore complex. Neostigmine may act on the ACh-receptor (the binding site of ACh) while physostigmine may interact with the ACh-gated cation channels.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Inoue M., Krishtal O. A. 'Concentration-clamp' study of gamma-aminobutyric-acid-induced chloride current kinetics in frog sensory neurones. J Physiol. 1986 Oct;379:171–185. doi: 10.1113/jphysiol.1986.sp016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Sokoll M. D., Sonesson B., Thesleff S. Studies on the nature of the cholinergic receptor. Eur J Pharmacol. 1968 Aug;4(1):40–46. doi: 10.1016/0014-2999(68)90007-1. [DOI] [PubMed] [Google Scholar]
- Andreev A. A., Veprintsev B. N., Vulfius C. A. Two-component desensitization of nicotinic receptors induced by acetylcholine agonists in Lymnaea stagnalis neurones. J Physiol. 1984 Aug;353:375–391. doi: 10.1113/jphysiol.1984.sp015341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter D. O., Greene L. A., Shain W., Vogel Z. Effects of eserine and neostigmine on the interaction of alpha-bungarotoxin with Aplysia acetylcholine receptors. Mol Pharmacol. 1976 Nov;12(6):999–1006. [PubMed] [Google Scholar]
- Fiekers J. F. Concentration-dependent effects of neostigmine on the endplate acetylcholine receptor channel complex. J Neurosci. 1985 Feb;5(2):502–514. doi: 10.1523/JNEUROSCI.05-02-00502.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K., Akaike N., Oomura Y., Kuraoka S. Internal perfusion studies demonstrating GABA-induced chloride responses in frog primary afferent neurons. Am J Physiol. 1984 Mar;246(3 Pt 1):C259–C265. doi: 10.1152/ajpcell.1984.246.3.C259. [DOI] [PubMed] [Google Scholar]
- Ishizuka S., Hattori K., Akaike N. Separation of ionic currents in the somatic membrane of frog sensory neurons. J Membr Biol. 1984;78(1):19–28. doi: 10.1007/BF01872528. [DOI] [PubMed] [Google Scholar]
- Kuba K., Albuquerque E. X., Barnard E. A. Diisopropylfluorophosphate: suppression of ionic conductance of the cholinergic receptor. Science. 1973 Aug 31;181(4102):853–856. doi: 10.1126/science.181.4102.853. [DOI] [PubMed] [Google Scholar]
- Kuba K., Nishi S. Characteristics of fast excitatory postsynaptic current in bullfrog sympathetic ganglion cells. Effects of membrane potential, temperature and Ca ions. Pflugers Arch. 1979 Jan 31;378(3):205–212. doi: 10.1007/BF00592737. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Connor E. A., Dionne V. E., Parsons R. L. Voltage clamp study of fast excitatory synaptic currents in bullfrog sympathetic ganglion cells. J Gen Physiol. 1980 Jan;75(1):39–60. doi: 10.1085/jgp.75.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono J. K., Salvaterra P. M. Snake alpha-toxin effects on cholinergic and noncholinergic responses of Aplysia californica neurons. J Neurosci. 1981 Mar;1(3):259–270. doi: 10.1523/JNEUROSCI.01-03-00259.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascuzzo G. J., Akaike A., Maleque M. A., Shaw K. P., Aronstam R. S., Rickett D. L., Albuquerque E. X. The nature of the interactions of pyridostigmine with the nicotinic acetylcholine receptor-ionic channel complex. I. Agonist, desensitizing, and binding properties. Mol Pharmacol. 1984 Jan;25(1):92–101. [PubMed] [Google Scholar]
- Shain W., Greene L. A., Carpenter D. O., Sytkowski A. J., Vogel Z. Aplysia acetylcholine receptors: blockade by and binding of alpha-bungarotoxin. Brain Res. 1974 Jun 7;72(2):225–240. doi: 10.1016/0006-8993(74)90861-0. [DOI] [PubMed] [Google Scholar]
- Slater N. T., Filbert M., Carpenter D. O. Multiple interactions of anticholinesterases with Aplysia acetylcholine responses. Brain Res. 1986 Jun 11;375(2):407–412. doi: 10.1016/0006-8993(86)90768-7. [DOI] [PubMed] [Google Scholar]
- Slater N. T., Hall A. F., Carpenter D. O. Kinetic properties of cholinergic desensitization in Aplysia neurons. Proc R Soc Lond B Biol Sci. 1984 Nov 22;223(1230):63–78. doi: 10.1098/rspb.1984.0083. [DOI] [PubMed] [Google Scholar]


