Abstract
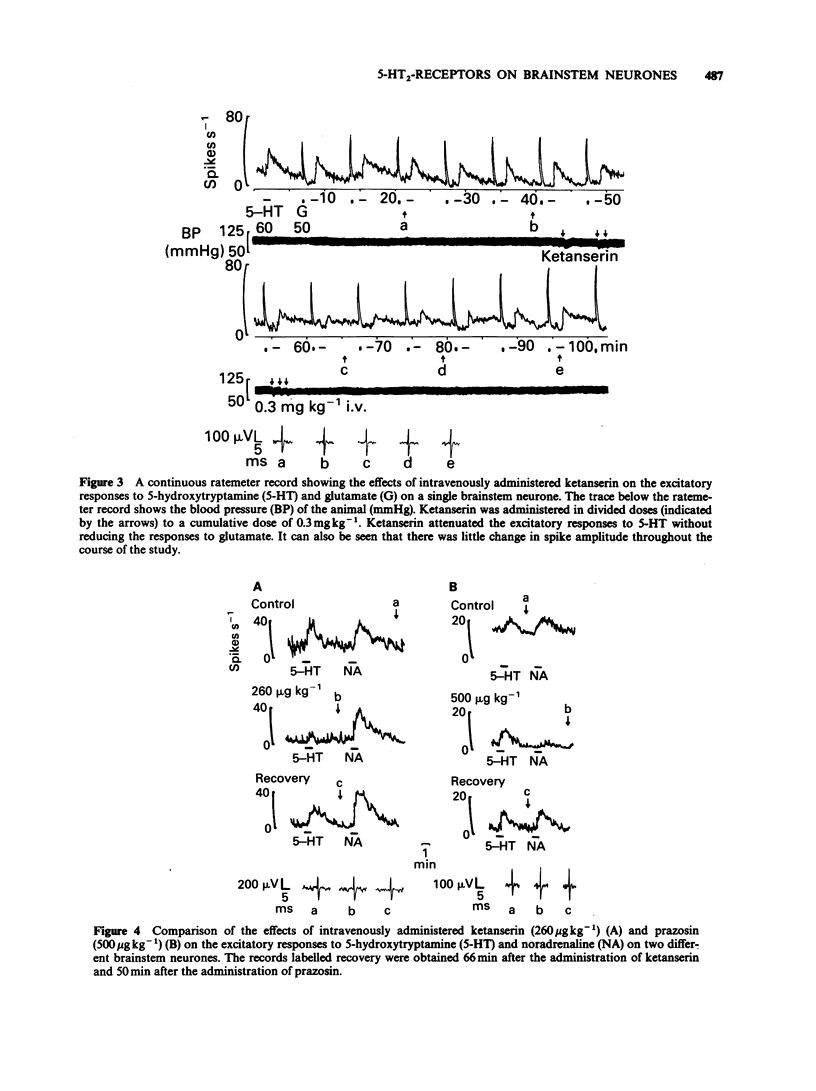
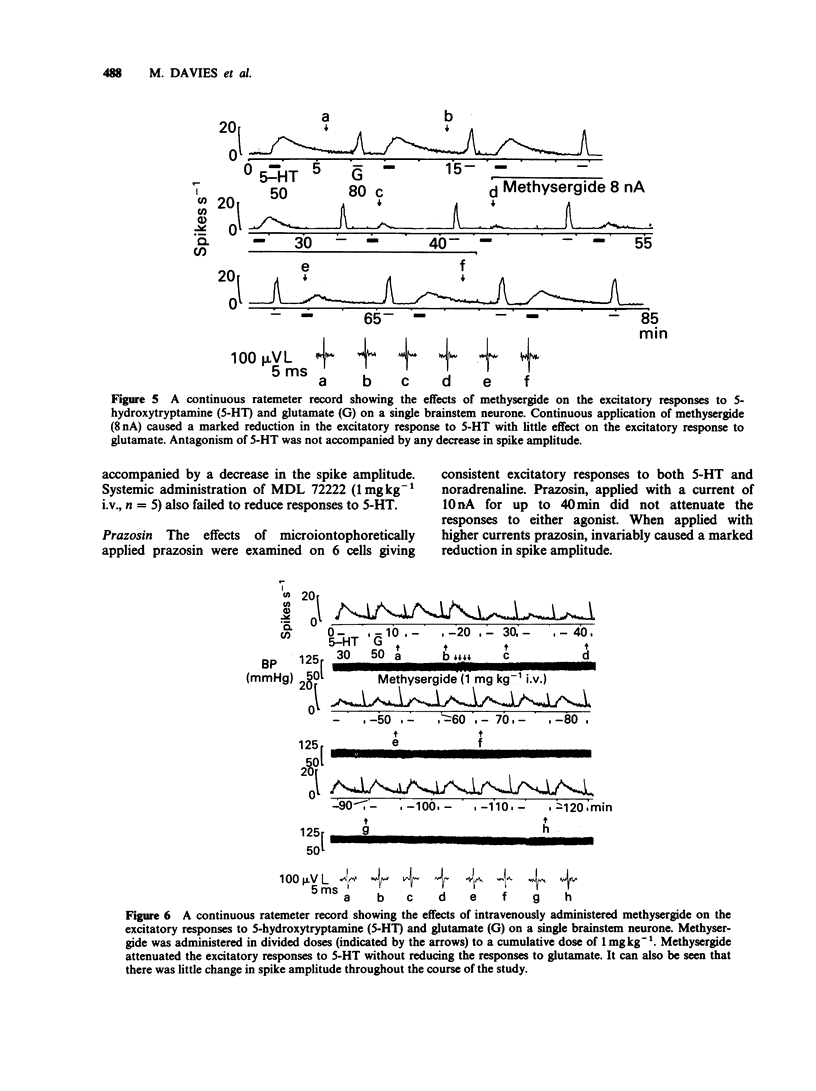
1. The technique of microiontophoresis was used to investigate the identity of the receptor mediating the excitatory effects of 5-hydroxytryptamine (5-HT) upon neurones in the midline of the medullary brainstem of the rat in vivo. 2. The 5-HT1-like receptor agonists 5-carboxamidotryptamine (5-CT) and 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) failed to excite the majority of neurones excited by 5-HT. The mobilities of 5-CT and 8-OH-DPAT when tested in vitro were found not to differ significantly from that of 5-HT, suggesting that the lack of effect of these agonists was not due to a lower rate of release from the microelectrodes. 3. The excitatory responses to 5-HT were attenuated by the 5-HT 2-receptor antagonists ketanserin and methysergide when applied microiontophoretically or administered intravenously (0.3 and 1 mg kg-1 respectively). Excitatory responses to glutamate and noradrenaline were not reduced. 4. The 5-HT3-receptor antagonist MDL 72222 failed to attenuate selectively the excitatory response to 5-HT when applied either by microiontophoresis or administered intravenously (1 mg kg-1). 5. Microiontophoretic application of the alpha 1-adrenoceptor antagonist prazosin did not attenuate excitatory responses to either 5-HT or noradrenaline. Intravenously administered prazosin (0.8 mg kg-1) also failed to attenuate excitatory responses to 5-HT, but did block excitatory responses to noradrenaline. 6. These results suggest that 5-HT2-receptors, but not 5-HT1-like receptors, 5-HT3-receptors or alpha 1-adrenoceptors, are involved in the excitatory response of midline medullary neurones to 5-HT.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apperley E., Humphrey P. P., Levy G. P. Receptors for 5-hydroxytryptamine and noradrenaline in rabbit isolated ear artery and aorta. Br J Pharmacol. 1976 Oct;58(2):211–221. doi: 10.1111/j.1476-5381.1976.tb10398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbehani M. M., Pomeroy S. L., Mack C. E. Interaction between central gray and nucleus raphe magnus: role of norepinephrine. Brain Res Bull. 1981 May;6(5):361–364. doi: 10.1016/s0361-9230(81)80004-4. [DOI] [PubMed] [Google Scholar]
- Black J. L., French R. J., Mylecharane E. J. Receptor mechanisms for 5-hydroxytryptamine in rabbit arteries. Br J Pharmacol. 1981 Nov;74(3):619–626. doi: 10.1111/j.1476-5381.1981.tb10472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes R. J., Bradley P. B., Briggs I., Dray A. Antagonism of 5-hydroxytryptamine by LSD 25 in the central nervous system: a possible neuronal basis for the actions of LSD 25. Br J Pharmacol. 1970 Oct;40(2):202–218. doi: 10.1111/j.1476-5381.1970.tb09914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes R. J., Bradley P. B., Brookes N., Candy J. M., Wolstencroft J. H. Actions of noradrenaline, other sympathomimetic amines and antagonists on neurones in the brain stem of the cat. Br J Pharmacol. 1971 Mar;41(3):462–479. doi: 10.1111/j.1476-5381.1971.tb08044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley P. B., Engel G., Feniuk W., Fozard J. R., Humphrey P. P., Middlemiss D. N., Mylecharane E. J., Richardson B. P., Saxena P. R. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology. 1986 Jun;25(6):563–576. doi: 10.1016/0028-3908(86)90207-8. [DOI] [PubMed] [Google Scholar]
- Bradshaw C. M., Pun R. Y., Slater N. T., Szabadi E. A procedure for comparing the mobilities of unlabeled drugs used in microelectrophoresis experiments. J Pharmacol Methods. 1981 Jan;5(1):67–73. doi: 10.1016/0160-5402(81)90104-2. [DOI] [PubMed] [Google Scholar]
- Bradshaw C. M., Roberts M. H., Szabadi E. Effects of imipramine and desipramine on responses of single cortical neurones to noradrenaline and 5-hydroxytryptamine. Br J Pharmacol. 1974 Nov;52(3):349–358. doi: 10.1111/j.1476-5381.1974.tb08602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw C. M., Roberts M. H., Szabadi E. Kinetics of the release of noradrenaline from micropipettes: interaction between ejecting and retaining currents. Br J Pharmacol. 1973 Dec;49(4):667–677. doi: 10.1111/j.1476-5381.1973.tb08543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W. The action of procaine and atropine on spinal neurones. J Physiol. 1960 Aug;153:17–34. doi: 10.1113/jphysiol.1960.sp006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton K. G., Bond R. A., Clarke D. E. An inhibitory prejunctional 5-HT1-like receptor in the isolated perfused rat kidney. Apparent distinction from the 5-HT1A, 5-HT1B and 5-HT1C subtypes. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):8–15. doi: 10.1007/BF00633190. [DOI] [PubMed] [Google Scholar]
- Couch J. R. Further evidence for a possible excitatory serotonergic synapse on raphe neurons of pons and lower midbrain. Life Sci. 1976 Sep 1;19(5):761–767. doi: 10.1016/0024-3205(76)90175-2. [DOI] [PubMed] [Google Scholar]
- Couch J. R., Jr Responses of neurons in the raphe nuclei to serotonin, norepinephrine and acetylcholine and their correlation with an excitatory synaptic input. Brain Res. 1970 Apr 1;19(1):137–150. doi: 10.1016/0006-8993(70)90243-x. [DOI] [PubMed] [Google Scholar]
- Engel G., Göthert M., Hoyer D., Schlicker E., Hillenbrand K. Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):1–7. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- Feniuk W., Humphrey P. P., Perren M. J., Watts A. D. A comparison of 5-hydroxytryptamine receptors mediating contraction in rabbit aorta and dog saphenous vein: evidence for different receptor types obtained by use of selective agonists and antagonists. Br J Pharmacol. 1985 Nov;86(3):697–704. doi: 10.1111/j.1476-5381.1985.tb08948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozard J. R. MDL 72222: a potent and highly selective antagonist at neuronal 5-hydroxytryptamine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1984 May;326(1):36–44. doi: 10.1007/BF00518776. [DOI] [PubMed] [Google Scholar]
- Fozard J. R. Mechanism of the hypotensive effect of ketanserin. J Cardiovasc Pharmacol. 1982 Sep-Oct;4(5):829–838. doi: 10.1097/00005344-198209000-00020. [DOI] [PubMed] [Google Scholar]
- Green A. R. 5-HT-mediated behavior. Animal studies. Neuropharmacology. 1984 Dec;23(12B):1521–1528. doi: 10.1016/0028-3908(84)90096-0. [DOI] [PubMed] [Google Scholar]
- Haigler H. J., Aghajanian G. K. Peripheral serotonin antagonists: failure to antagonize serotonin in brain areas receiving a prominent serotonergic input. J Neural Transm. 1974;35(4):157–273. doi: 10.1007/BF02205223. [DOI] [PubMed] [Google Scholar]
- Johnson E. S., Roberts M. H., Sobieszek A., Straughan D. W. Noradrenaline sensitive cells in cat cerebral cortex. Int J Neuropharmacol. 1969 Dec;8(6):549–566. doi: 10.1016/0028-3908(69)90072-0. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Awouters F., Kennis L., Laduron P. M., Vandenberk J., Janssen P. A. Receptor binding profile of R 41 468, a novel antagonist at 5-HT2 receptors. Life Sci. 1981 Mar 2;28(9):1015–1022. doi: 10.1016/0024-3205(81)90747-5. [DOI] [PubMed] [Google Scholar]
- McCall R. B., Aghajanian G. K. Pharmacological characterization of serotonin receptors in the facial motor nucleus: a microiontophoretic study. Eur J Pharmacol. 1980 Jul 25;65(2-3):175–183. doi: 10.1016/0014-2999(80)90390-8. [DOI] [PubMed] [Google Scholar]
- McCall R. B., Schuette M. R. Evidence for an alpha-1 receptor-mediated central sympathoinhibitory action of ketanserin. J Pharmacol Exp Ther. 1984 Mar;228(3):704–710. [PubMed] [Google Scholar]
- Middlemiss D. N., Fozard J. R. 8-Hydroxy-2-(di-n-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur J Pharmacol. 1983 May 20;90(1):151–153. doi: 10.1016/0014-2999(83)90230-3. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Lebovitz R. M., Snyder S. H. Two distinct central serotonin receptors with different physiological functions. Science. 1981 May 15;212(4496):827–829. doi: 10.1126/science.7221567. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Snyder S. H. Multiple serotonin receptors: differential binding of [3H]5-hydroxytryptamine, [3H]lysergic acid diethylamide and [3H]spiroperidol. Mol Pharmacol. 1979 Nov;16(3):687–699. [PubMed] [Google Scholar]
- Roberts M. H., Straughan D. W. Excitation and depression of cortical neurones by 5-hydroxytryptamine. J Physiol. 1967 Nov;193(2):269–294. doi: 10.1113/jphysiol.1967.sp008357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevethick M. A., Feniuk W., Humphrey P. P. 5-Carboxamidotryptamine: a potent agonist mediating relaxation and elevation of cyclic AMP in the isolated neonatal porcine vena cava. Life Sci. 1986 Apr 21;38(16):1521–1528. doi: 10.1016/0024-3205(86)90566-7. [DOI] [PubMed] [Google Scholar]
- Yap C. Y., Taylor D. A. Involvement of 5-HT2 receptors in the wet-dog shake behaviour induced by 5-hydroxytryptophan in the rat. Neuropharmacology. 1983 Jul;22(7):801–804. doi: 10.1016/0028-3908(83)90123-5. [DOI] [PubMed] [Google Scholar]


