Abstract
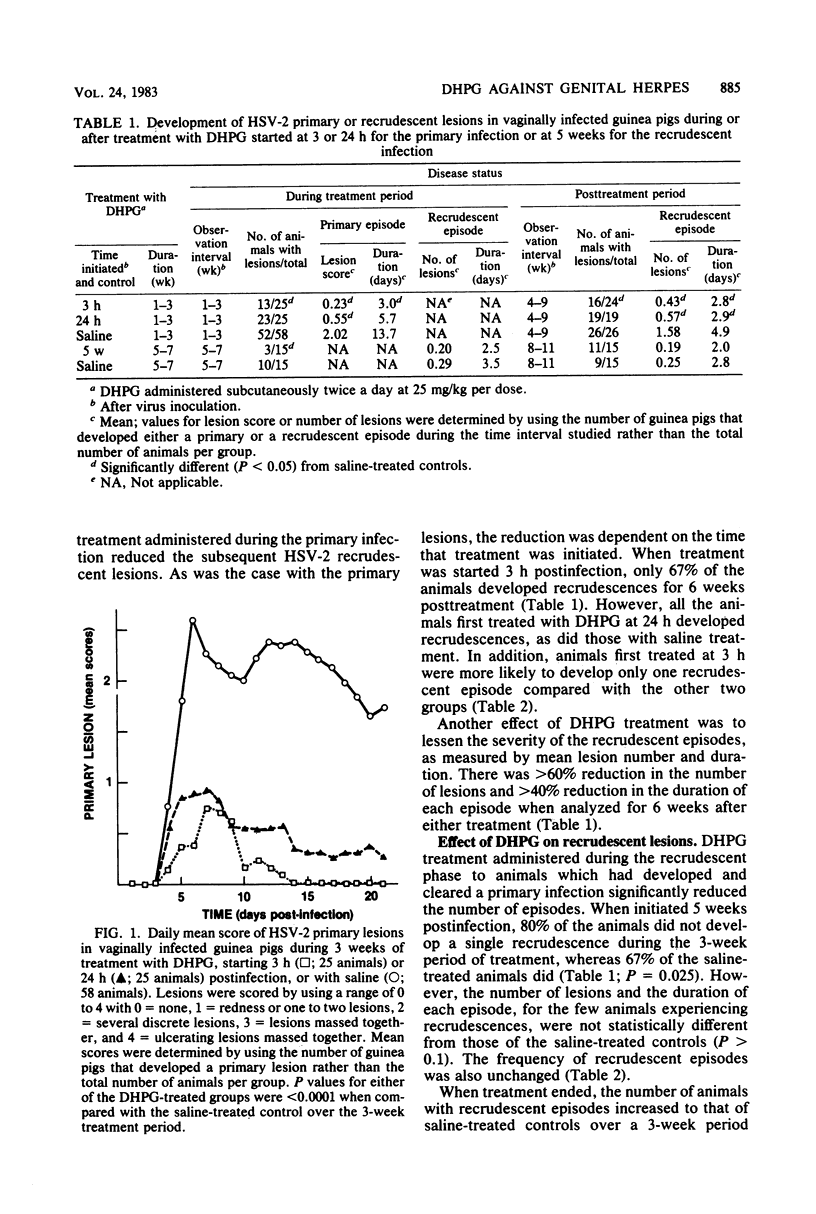
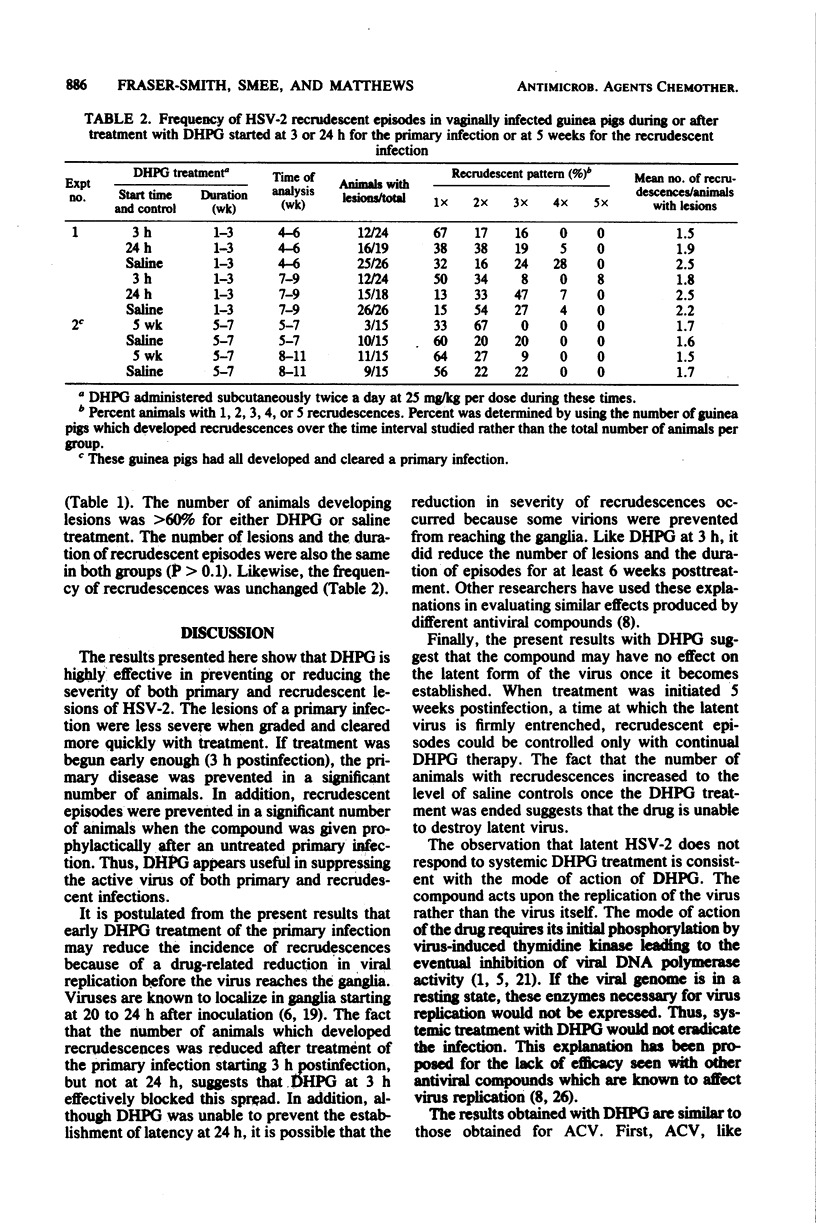
The acyclic nucleoside 9-(1,3-dihydroxy-2-propoxymethyl)guanine (DHPG) was evaluated for its efficacy in protecting guinea pigs from primary and recrudescent infections of herpes simplex virus type 2. Vaginally infected guinea pigs were treated twice a day with DHPG at 25 mg/kg per dose for 3 weeks. Subcutaneous doses were started 3 h, 24 h, or 5 weeks after virus inoculation. Treatment starting at 3 or 24 h reduced the severity of the primary infection by greater than 70% when lesions were graded for 3 weeks; lesion duration was lessened by greater than 55%. For 6 weeks after treatment, the number of recrudescent lesions was reduced by greater than 60%, and the duration of the recrudescences declined by greater than 40%. When dosing was started at 3 h postinfection, 33% of the animals did not develop any sign of primary or recrudescent infection throughout the 9-week test. By comparison, all the animals treated with DHPG starting at 24 h or with saline became infected. A 3-week DHPG regimen starting 5 weeks postinfection reduced the number of animals that developed recrudescent lesions by 70%. When treatment ended, however, recrudescent episodes in the animals increased to the level of saline-treated controls.These results suggest that (i) DHPG is highly effective in reducing the severity of both primary and recrudescent lesions of herpes simplex virus type 2, (ii) early treatment of a primary infection or treatment of recrudescences reduces the incidence of recrudescences, and (iii) the drug appears to have no effect on the latent form of the virus, as the incidence of recrudescences increases when DHPG treatment is ended.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton W. T., Karkas J. D., Field A. K., Tolman R. L. Activation by thymidine kinase and potent antiherpetic activity of 2'-nor-2'-deoxyguanosine (2'NDG). Biochem Biophys Res Commun. 1982 Oct 29;108(4):1716–1721. doi: 10.1016/s0006-291x(82)80109-5. [DOI] [PubMed] [Google Scholar]
- Baringer J. R. Recovery of herpes simplex virus from human sacral ganglions. N Engl J Med. 1974 Oct 17;291(16):828–830. doi: 10.1056/NEJM197410172911606. [DOI] [PubMed] [Google Scholar]
- Baringer J. R., Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973 Mar 29;288(13):648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Huang E. S., Lin J. C., Mar E. C., Pagano J. S., Dutschman G. E., Grill S. P. Unique spectrum of activity of 9-[(1,3-dihydroxy-2-propoxy)methyl]-guanine against herpesviruses in vitro and its mode of action against herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1983 May;80(9):2767–2770. doi: 10.1073/pnas.80.9.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973 Feb;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Bell S. E., Elion G. B., Nash A. A., Wildy P. Effect of acycloguanosine treatment of acute and latent herpes simplex infections in mice. Antimicrob Agents Chemother. 1979 Apr;15(4):554–561. doi: 10.1128/aac.15.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Wildy P. Recurrent herpes simplex: the outlook for systemic antiviral agents. Br Med J (Clin Res Ed) 1981 Jun 6;282(6279):1821–1822. doi: 10.1136/bmj.282.6279.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern E. R. Acyclovir treatment of experimental genital herpes simplex virus infections. Am J Med. 1982 Jul 20;73(1A):100–108. doi: 10.1016/0002-9343(82)90073-0. [DOI] [PubMed] [Google Scholar]
- Kern E. R., Glasgow L. A., Overall J. C., Jr, Reno J. M., Boezi J. A. Treatment of experimental herpesvirus infections with phosphonoformate and some comparisons with phosphonoacetate. Antimicrob Agents Chemother. 1978 Dec;14(6):817–823. doi: 10.1128/aac.14.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. J., Friedman-Kien A. E., DeStefano E. Latent herpes simplex virus infections in sensory ganglia of hairless mice prevented by acycloguanosine. Antimicrob Agents Chemother. 1979 May;15(5):723–729. doi: 10.1128/aac.15.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. C., Dvorak C. A., Smee D. F., Matthews T. R., Verheyden J. P. 9-[(1,3-Dihydroxy-2-propoxy)methyl]guanine: a new potent and selective antiherpes agent. J Med Chem. 1983 May;26(5):759–761. doi: 10.1021/jm00359a023. [DOI] [PubMed] [Google Scholar]
- Openshaw H., Puga A., Notkins A. L. Herpes simplex virus infection in sensory ganglia: immune control, latency, and reactivation. Fed Proc. 1979 Dec;38(13):2660–2664. [PubMed] [Google Scholar]
- Park N. H., Pavan-Langston D., Hettinger M. E., McLean S. L., Albert D. M., Lin T. S., Prusoff W. H. Topical therapeutic efficacy of 9-(2-hydroxyethoxymethyl)guanine and 5-iodo-5'-amino-2',5'-dideoxyuridine on oral infection with herpes simplex virus in mice. J Infect Dis. 1980 May;141(5):575–579. doi: 10.1093/infdis/141.5.575. [DOI] [PubMed] [Google Scholar]
- Pronovost A. D., Lucia H. L., Dann P. R., Hsiung G. D. Effect of acyclovir on genital herpes in guinea pigs. J Infect Dis. 1982 Jun;145(6):904–908. doi: 10.1093/infdis/145.6.904. [DOI] [PubMed] [Google Scholar]
- Puga A., Rosenthal J. D., Openshaw H., Notkins A. L. Herpes simplex virus DNA and mRNA sequences in acutely and chronically infected trigeminal ganglia of mice. Virology. 1978 Aug;89(1):102–111. doi: 10.1016/0042-6822(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Rollinson E. A., White G. Relative activities of acyclovir and BW759 against Aujeszky's disease and equine rhinopneumonitis viruses. Antimicrob Agents Chemother. 1983 Aug;24(2):221–226. doi: 10.1128/aac.24.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriba M. Recurrent genital Herpes simplex virus (HSV) infection of guinea pigs. Med Microbiol Immunol. 1976 Dec 1;162(3-4):201–208. doi: 10.1007/BF02120998. [DOI] [PubMed] [Google Scholar]
- Smee D. F., Martin J. C., Verheyden J. P., Matthews T. R. Anti-herpesvirus activity of the acyclic nucleoside 9-(1,3-dihydroxy-2-propoxymethyl)guanine. Antimicrob Agents Chemother. 1983 May;23(5):676–682. doi: 10.1128/aac.23.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. O., Galloway K. S., Kennell W. L., Ogilvie K. K., Radatus B. K. A new nucleoside analog, 9-[[2-hydroxy-1-(hydroxymethyl)ethoxyl]methyl]guanine, highly active in vitro against herpes simplex virus types 1 and 2. Antimicrob Agents Chemother. 1982 Jul;22(1):55–61. doi: 10.1128/aac.22.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanberry L. R., Kern E. R., Richards J. T., Abbott T. M., Overall J. C., Jr Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. J Infect Dis. 1982 Sep;146(3):397–404. doi: 10.1093/infdis/146.3.397. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Svennerholm B., Vahlne A., Lycke E. Persistent reactivable latent herpes simplex virus infection in trigeminal ganglia of mice treated with antiviral drugs. Arch Virol. 1981;69(1):43–48. doi: 10.1007/BF01315264. [DOI] [PubMed] [Google Scholar]
- Walz M. A., Price R. W., Notkins A. L. Latent ganglionic infection with herpes simplex virus types 1 and 2: viral reactivation in vivo after neurectomy. Science. 1974 Jun 14;184(4142):1185–1187. doi: 10.1126/science.184.4142.1185. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Walz M. A., Notkins A. L. Viral-specific thymidine kinase in sensory ganglia of mice infected with herpes simplex virus. Virology. 1977 Feb;76(2):866–869. doi: 10.1016/0042-6822(77)90267-7. [DOI] [PubMed] [Google Scholar]


