Abstract
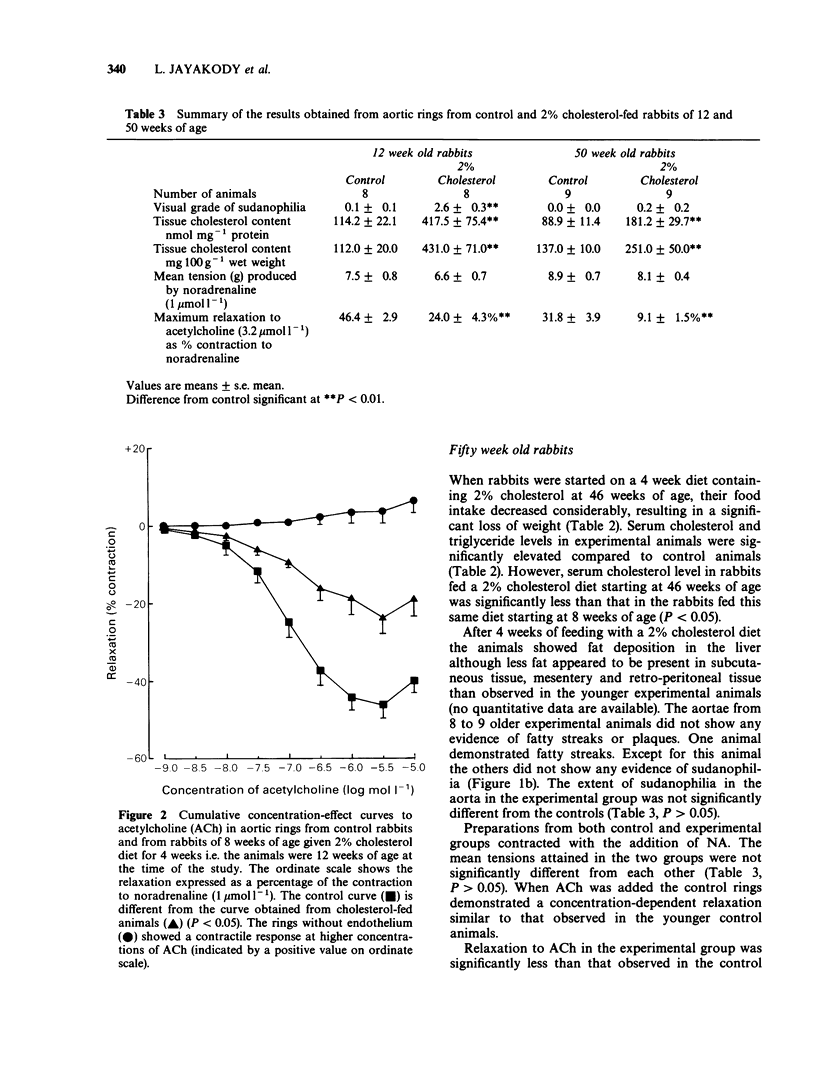
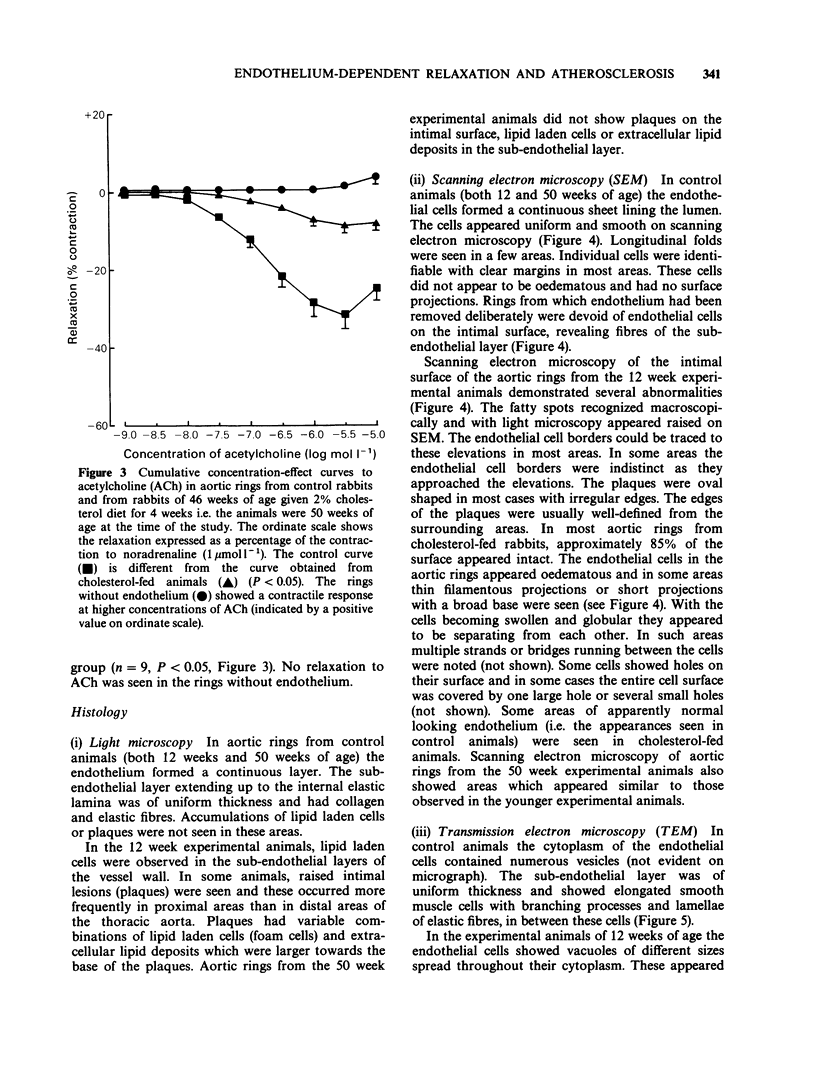
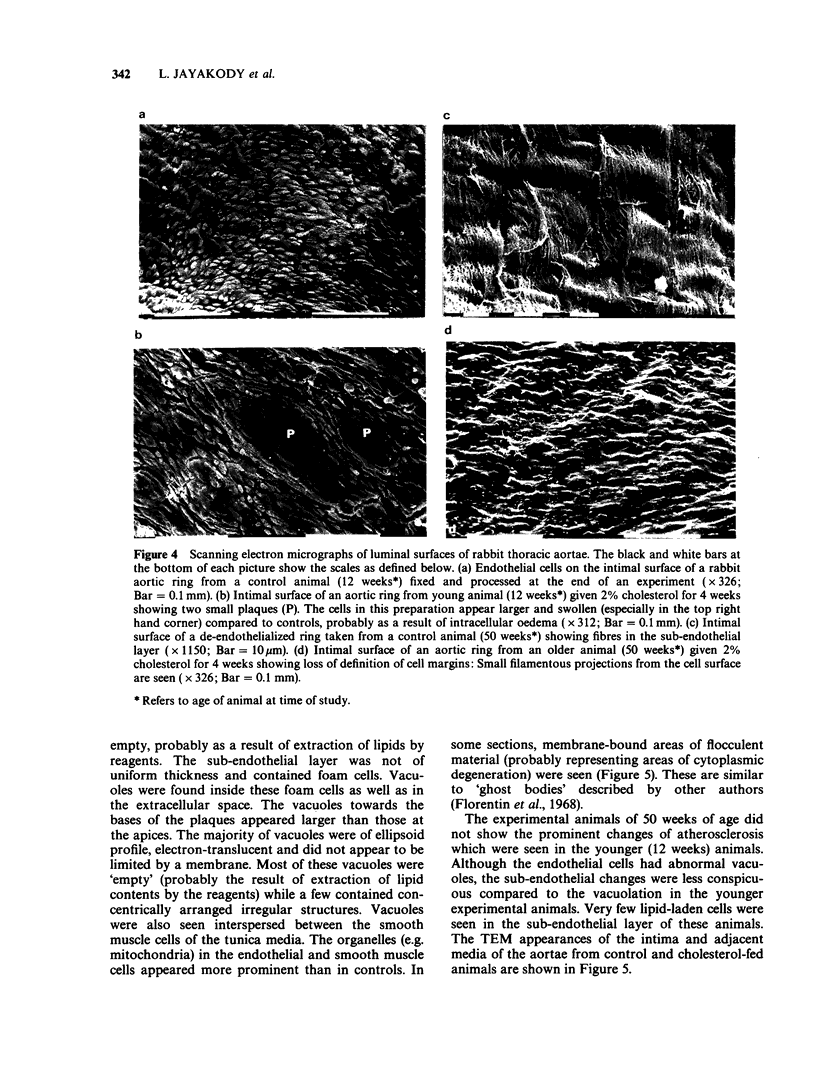
1. Cholesterol feeding of rabbits impairs the endothelium-dependent relaxation (EDR) evoked by acetylcholine (ACh) in the aorta. The experiments described in this paper were undertaken to examine the influence of age upon this phenomenon. 2. Rabbits aged 8 weeks and 46 weeks were fed a diet containing 2% cholesterol and other lipids for 4 weeks. Age-matched control animals were fed a standard rabbit diet. The concentrations of cholesterol and triglycerides in plasma were measured and the extent of atherosclerosis was estimated by staining the aortae with Sudan Red. Light and electron microscopy were undertaken also. 3. Rings of aorta were prepared for recording isometric tension. They were contracted with noradrenaline (NA) and EDR elicited by adding ACh. 4. The young rabbits showed weight gain, hypercholesterolaemia, prominent Sudan Red staining, together with scanning and transmission electron microscopic (SEM and TEM) features of cholesterol-induced atherosclerosis. The older animals showed significant weight loss and hypercholesterolaemia. The aortae of these animals showed no significant sudanophilia or light microscopic features of atherosclerosis. The SEM appearances were similar to the young animals fed cholesterol. 5. EDR to ACh was significantly impaired in both groups of cholesterol-fed rabbits. The maximal relaxations to ACh in young control and cholesterol-fed rabbits were 46.4 +/- 2.9% and 24.0 +/- 4.3% (mean +/- s.e. mean, n = 8, P less than 0.05) of the contractile response to NA (1 mumol 1(-1]. The corresponding results in the age control and cholesterol-fed rabbits were 31.8 +/- 3.9% and 9.1 +/- 1.5% (n = 9, P less than 0.05). 6. The young rabbits were far more susceptible to cholesterol-induced atherosclerosis than older animals and these changes were accompanied by loss of EDR. In the older animals and these changes were accompanied by loss of EDR. In the older animals the loss of the latter property was not accompanied by a significant degree of atherosclerosis although hypercholesterolaemia was present.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- Allessie M. A., Lammers W. J., Bonke I. M., Hollen J. Intra-atrial reentry as a mechanism for atrial flutter induced by acetylcholine and rapid pacing in the dog. Circulation. 1984 Jul;70(1):123–135. doi: 10.1161/01.cir.70.1.123. [DOI] [PubMed] [Google Scholar]
- Bossaller C., Habib G. B., Yamamoto H., Williams C., Wells S., Henry P. D. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5'-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J Clin Invest. 1987 Jan;79(1):170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell S. P., Lewis M. J., Henderson A. H. Effect of lipid feeding on endothelium dependent relaxation in rabbit aortic preparations. Cardiovasc Res. 1987 Jan;21(1):34–38. doi: 10.1093/cvr/21.1.34. [DOI] [PubMed] [Google Scholar]
- Cherry P. D., Furchgott R. F., Zawadzki J. V., Jothianandan D. Role of endothelial cells in relaxation of isolated arteries by bradykinin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2106–2110. doi: 10.1073/pnas.79.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUFF G. L., McMILLAN G. C., RITCHIE A. C. The morphology of early atherosclerotic lesions of the aorta demonstrated by the surface technique in rabbits fed cholesterol; together with a description of the anatomy of the intima of the rabbit's aorta and the spontaneous lesions which occur in it. Am J Pathol. 1957 Sep-Oct;33(5):845–873. [PMC free article] [PubMed] [Google Scholar]
- Florentin R. A., Nam S. C., Daoud A. S., Jones R., Scott R. F., Morrison E. S., Kim D. N., Lee K. T., Thomas W. A., Dodds W. J. Dietary-induced atherosclerosis in miniature swine. Exp Mol Pathol. 1968 Jun;8(3):263–301. doi: 10.1016/s0014-4800(68)80001-2. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- HOLMAN R. L., McGILL H. C., Jr, STRONG J. P., GEER J. C. Technics for studying atherosclerotic lesions. Lab Invest. 1958 Jan-Feb;7(1):42–47. [PubMed] [Google Scholar]
- Jayakody L., Senaratne M., Thomson A., Kappagoda T. Endothelium-dependent relaxation in experimental atherosclerosis in the rabbit. Circ Res. 1987 Feb;60(2):251–264. doi: 10.1161/01.res.60.2.251. [DOI] [PubMed] [Google Scholar]
- KRITCHEVSKY D., MOYNIHAN J. L., LANGAN J., TEPPER S. A., SACHS M. L. Effect of D- and L-thyroxine and of D- and L-3,5,3'-triiodothyronine on development and regression of experimental atherosclerosis in rabbits. J Atheroscler Res. 1961 May-Jun;1:211–221. doi: 10.1016/s0368-1319(61)80032-x. [DOI] [PubMed] [Google Scholar]
- Kalsner S. Cholinergic mechanisms in human coronary artery preparations: implications of species differences. J Physiol. 1985 Jan;358:509–526. doi: 10.1113/jphysiol.1985.sp015564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin R. J. Rapid enzymatic determination of free and esterified cholesterol content of serum and tissues. Clin Chim Acta. 1976 Aug 16;71(1):75–80. doi: 10.1016/0009-8981(76)90277-1. [DOI] [PubMed] [Google Scholar]
- Pinter J. K., Hayashi J. A., Watson J. A. Enzymic assay of glycerol, dihydroxyacetone, and glyceraldehyde. Arch Biochem Biophys. 1967 Aug;121(2):404–414. doi: 10.1016/0003-9861(67)90094-x. [DOI] [PubMed] [Google Scholar]
- Senaratne M., Kappagoda T. Tetrodotoxin-resistant relaxation to transmural nerve stimulation in isolated saphenous vein. Am J Physiol. 1984 Dec;247(6 Pt 2):H952–H959. doi: 10.1152/ajpheart.1984.247.6.H952. [DOI] [PubMed] [Google Scholar]
- Shepherd J. T., Vanhoutte P. M. Spasm of the coronary arteries: causes and consequences (the scientist's viewpoint). Mayo Clin Proc. 1985 Jan;60(1):33–46. doi: 10.1016/s0025-6196(12)65280-x. [DOI] [PubMed] [Google Scholar]
- Sreeharan N., Jayakody R. L., Senaratne M. P., Thomson A. B., Kappagoda C. T. Endothelium-dependent relaxation and experimental atherosclerosis in the rabbit aorta. Can J Physiol Pharmacol. 1986 Nov;64(11):1451–1453. doi: 10.1139/y86-246. [DOI] [PubMed] [Google Scholar]
- Swaner J. C., Connor W. E. Hypercholesterolemia of total starvation: its mechanism via tissue mobilization of cholesterol. Am J Physiol. 1975 Aug;229(2):365–369. doi: 10.1152/ajplegacy.1975.229.2.365. [DOI] [PubMed] [Google Scholar]
- Thomson A. B. Aging and cholesterol uptake in the rabbit jejunum: role of the bile salt micelle and the unstirred water layer. Dig Dis Sci. 1981 Oct;26(10):890–896. doi: 10.1007/BF01309492. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rimele T. J. Role of the endothelium in the control of vascular smooth muscle function. J Physiol (Paris) 1982;78(7):681–686. [PubMed] [Google Scholar]
- Verbeuren T. J., Jordaens F. H., Zonnekeyn L. L., Van Hove C. E., Coene M. C., Herman A. G. Effect of hypercholesterolemia on vascular reactivity in the rabbit. I. Endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986 Apr;58(4):552–564. doi: 10.1161/01.res.58.4.552. [DOI] [PubMed] [Google Scholar]
- West C. E., Deuring K., Schutte J. B., Terpstra A. H. The effect of age on the development of hypercholesterolemia in rabbits fed semipurified diets containing casein. J Nutr. 1982 Jul;112(7):1287–1295. doi: 10.1093/jn/112.7.1287. [DOI] [PubMed] [Google Scholar]





