Abstract
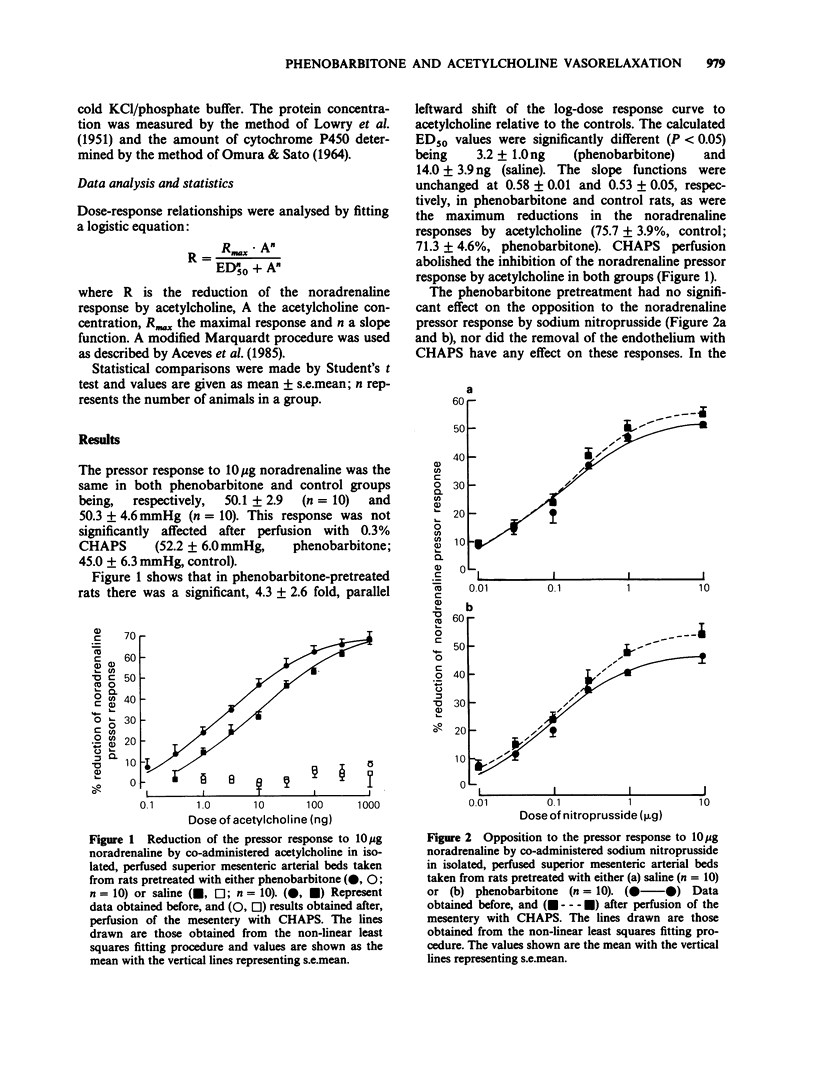
1. Pretreatment of rats for 5 days with phenobarbitone (80 mg kg-1 day-1) enhanced the potency enhanced the potency of acetylcholine in opposing noradrenaline-induced vasoconstriction in the isolated perfused superior mesenteric arterial bed; in 10 saline-pretreated control animals the ED50 was 14.0 +/- 3.9 ng whereas it was 3.23 +/- 1.00 ng in 10 phenobarbitone-pretreated animals. 2. In both saline- and phenobarbitone-pretreated rats acetylcholine was ineffective at opposing noradrenaline vasoconstriction after the mesentery had been perfused for 90s with a 0.3% solution of the detergent CHAPS in distilled water (to remove the endothelium), but pressor responses to noradrenaline were unaffected. 3. Pretreatment with phenobarbitone had no effect on the opposition by sodium nitroprusside of noradrenaline pressor responses. Also, the effects of nitroprusside were not affected by perfusion with CHAPS in either control or barbiturate-pretreated groups. 4. Inclusion of indomethacin (10 microM) in the perfusion fluid had no effect on the enhancement by phenobarbitone pretreatment of the endothelium-dependent opposition by acetylcholine of noradrenaline pressor responses; the ED50 values in the absence and presence of indomethacin were, respectively, 2.40 +/- 0.31 ng and 1.87 +/- 0.27 ng (n = 6). 5. The concentration of cytochrome P450 in the microsomal fraction obtained from the mesenteric preparation was increased from 204 +/- 32 (saline-pretreated; n = 7) to 784 +/- 249 pmol g-1 wet wt (n = 7) by the phenobarbitone pretreatment. 6. It is concluded that the increase in potency of acetylcholine as an endothelium-dependent vasodilator by phenobarbitone pretreatment is most probably at the level of the endothelium rather than the vascular smooth muscle.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N. G., Pinto A., Mullane K. M., Levere R. D., Spokas E. Presence of cytochrome P-450-dependent monooxygenase in intimal cells of the hog aorta. Hypertension. 1985 Nov-Dec;7(6 Pt 1):899–904. doi: 10.1161/01.hyp.7.6.899. [DOI] [PubMed] [Google Scholar]
- Aceves J., Mariscal S., Morrison K. E., Young J. M. The binding of doxepin to histamine H1-receptors in guinea-pig and rat brain. Br J Pharmacol. 1985 Feb;84(2):417–424. doi: 10.1111/j.1476-5381.1985.tb12925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J., Chacos N., Werringloer J., Prough R. A., Estabrook R. W. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5362–5366. doi: 10.1073/pnas.78.9.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. A., Schwartzman M., Capdevila J., Falck J. R., McGiff J. C. Vasoactivity of arachidonic acid epoxides. Eur J Pharmacol. 1987 Jun 19;138(2):281–283. doi: 10.1016/0014-2999(87)90445-6. [DOI] [PubMed] [Google Scholar]
- Chacos N., Falck J. R., Wixtrom C., Capdevila J. Novel epoxides formed during the liver cytochrome P-450 oxidation of arachidonic acid. Biochem Biophys Res Commun. 1982 Feb 11;104(3):916–922. doi: 10.1016/0006-291x(82)91336-5. [DOI] [PubMed] [Google Scholar]
- Chand N., Altura B. M. Acetylcholine and bradykinin relax intrapulmonary arteries by acting on endothelial cells: role in lung vascular diseases. Science. 1981 Sep 18;213(4514):1376–1379. doi: 10.1126/science.7268440. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Alheid U., Frölich J. C., Mülsch A. Mechanisms of action of lipoxygenase and cytochrome P-450-mono-oxygenase inhibitors in blocking endothelium-dependent vasodilatation. Br J Pharmacol. 1988 Mar;93(3):569–578. doi: 10.1111/j.1476-5381.1988.tb10312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstermann U., Burgwitz K., Frölich J. C. Thimerosal induces endothelium-dependent vascular smooth muscle relaxations by interacting with thiol groups. Relaxations are likely to be mediated by endothelium-derived relaxing factor (EDRF). Naunyn Schmiedebergs Arch Pharmacol. 1986 Dec;334(4):501–507. doi: 10.1007/BF00569393. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Goppelt-Strübe M., Frölich J. C., Busse R. Inhibitors of acyl-coenzyme A:lysolecithin acyltransferase activate the production of endothelium-derived vascular relaxing factor. J Pharmacol Exp Ther. 1986 Jul;238(1):352–359. [PubMed] [Google Scholar]
- Förstermann U., Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ Res. 1986 Apr;58(4):531–538. doi: 10.1161/01.res.58.4.531. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Neufang B. Endothelium-dependent vasodilation by melittin: are lipoxygenase products involved? Am J Physiol. 1985 Jul;249(1 Pt 2):H14–H19. doi: 10.1152/ajpheart.1985.249.1.H14. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Trogisch G., Busse R. Species-dependent differences in the nature of endothelium-derived vascular relaxing factor. Eur J Pharmacol. 1984 Nov 27;106(3):639–643. doi: 10.1016/0014-2999(84)90071-2. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Gruetter D. Y., Lyon J. E., Kadowitz P. J., Ignarro L. J. Relationship between cyclic guanosine 3':5'-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Pharmacol Exp Ther. 1981 Oct;219(1):181–186. [PubMed] [Google Scholar]
- Hiley C. R., Nichols A. J., Wilson A. C. Effects of phenobarbitone and 6-methylprednisolone pretreatment on pressure/flow relations in the superior mesenteric and iliac arterial beds of the rat. J Pharm Pharmacol. 1985 Mar;37(3):164–169. doi: 10.1111/j.2042-7158.1985.tb05033.x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Juchau M. R., Bond J. A., Benditt E. P. Aryl 4-monooxygenase and cytochrome P-450 in the aorta: possible role in atherosclerosis. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3723–3725. doi: 10.1073/pnas.73.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCGREGOR D. D. THE EFFECT OF SYMPATHETIC NERVE STIMULATION OF VASOCONSTRICTOR RESPONSES IN PERFUSED MESENTERIC BLOOD VESSELS OF THE RAT. J Physiol. 1965 Mar;177:21–30. doi: 10.1113/jphysiol.1965.sp007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A. R., Pascoe N. Metabolism of arachidonate through NADPH-dependent oxygenase of renal cortex. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7375–7378. doi: 10.1073/pnas.78.12.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Ohnhaus E. E., Thorgeirsson S. S., Davies D. S., Breckenridge A. Changes in liver blood flow during enzyme induction. Biochem Pharmacol. 1971 Oct;20(10):2561–2570. doi: 10.1016/0006-2952(71)90164-x. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pinto A., Abraham N. G., Mullane K. M. Arachidonic acid-induced endothelial-dependent relaxations of canine coronary arteries: contribution of a cytochrome P-450-dependent pathway. J Pharmacol Exp Ther. 1987 Mar;240(3):856–863. [PubMed] [Google Scholar]
- Pinto A., Abraham N. G., Mullane K. M. Cytochrome P-450-dependent monooxygenase activity and endothelial-dependent relaxations induced by arachidonic acid. J Pharmacol Exp Ther. 1986 Feb;236(2):445–451. [PubMed] [Google Scholar]
- Proctor K. G., Falck J. R., Capdevila J. Intestinal vasodilation by epoxyeicosatrienoic acids: arachidonic acid metabolites produced by a cytochrome P450 monooxygenase. Circ Res. 1987 Jan;60(1):50–59. doi: 10.1161/01.res.60.1.50. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Schwartzman M., Ferreri N. R., Carroll M. A., Songu-Mize E., McGiff J. C. Renal cytochrome P450-related arachidonate metabolite inhibits (Na+ + K+)ATPase. Nature. 1985 Apr 18;314(6012):620–622. doi: 10.1038/314620a0. [DOI] [PubMed] [Google Scholar]
- Shikano K., Ohlstein E. H., Berkowitz B. A. Differential selectivity of endothelium-derived relaxing factor and nitric oxide in smooth muscle. Br J Pharmacol. 1987 Nov;92(3):483–485. doi: 10.1111/j.1476-5381.1987.tb11347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer H. A., Saye J. A., Peach M. J. Effects of cytochrome P-450 inhibitors on endothelium-dependent relaxation in rabbit aorta. Blood Vessels. 1984;21(5):223–230. doi: 10.1159/000158515. [DOI] [PubMed] [Google Scholar]
- Spokas E. G., Folco G. C. Intima-related vasodilatation of the perfused rat caudal artery. Eur J Pharmacol. 1984 Apr 20;100(2):211–217. doi: 10.1016/0014-2999(84)90225-5. [DOI] [PubMed] [Google Scholar]
- Ullrich V., Castle L., Weber P. Spectral evidence for the cytochrome P450 nature of prostacyclin synthetase. Biochem Pharmacol. 1981 Jul 15;30(14):2033–2036. doi: 10.1016/0006-2952(81)90218-5. [DOI] [PubMed] [Google Scholar]
- Yates M. S., Hiley C. R., Roberts P. J., Back D. J., Crawford F. E. Differential effects of hepatic microsomal enzyme inducing agents on liver blood flow. Biochem Pharmacol. 1978;27(22):2617–2621. doi: 10.1016/0006-2952(78)90336-2. [DOI] [PubMed] [Google Scholar]


