Abstract
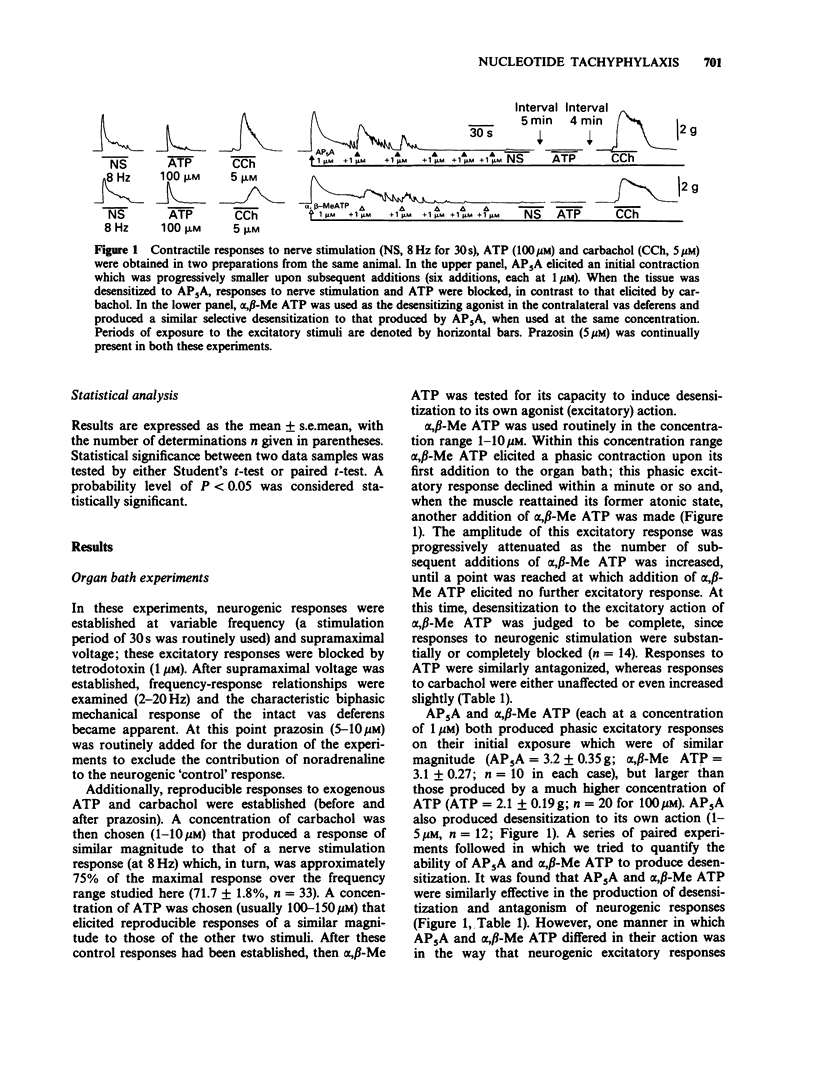
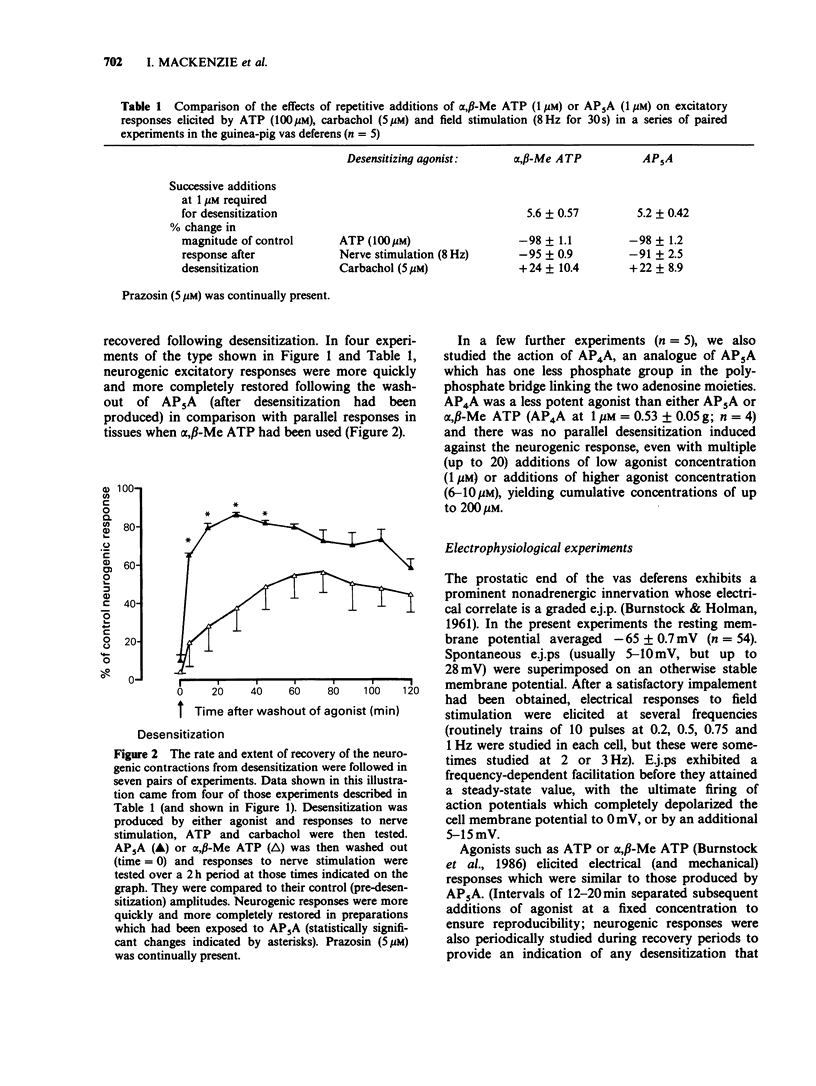
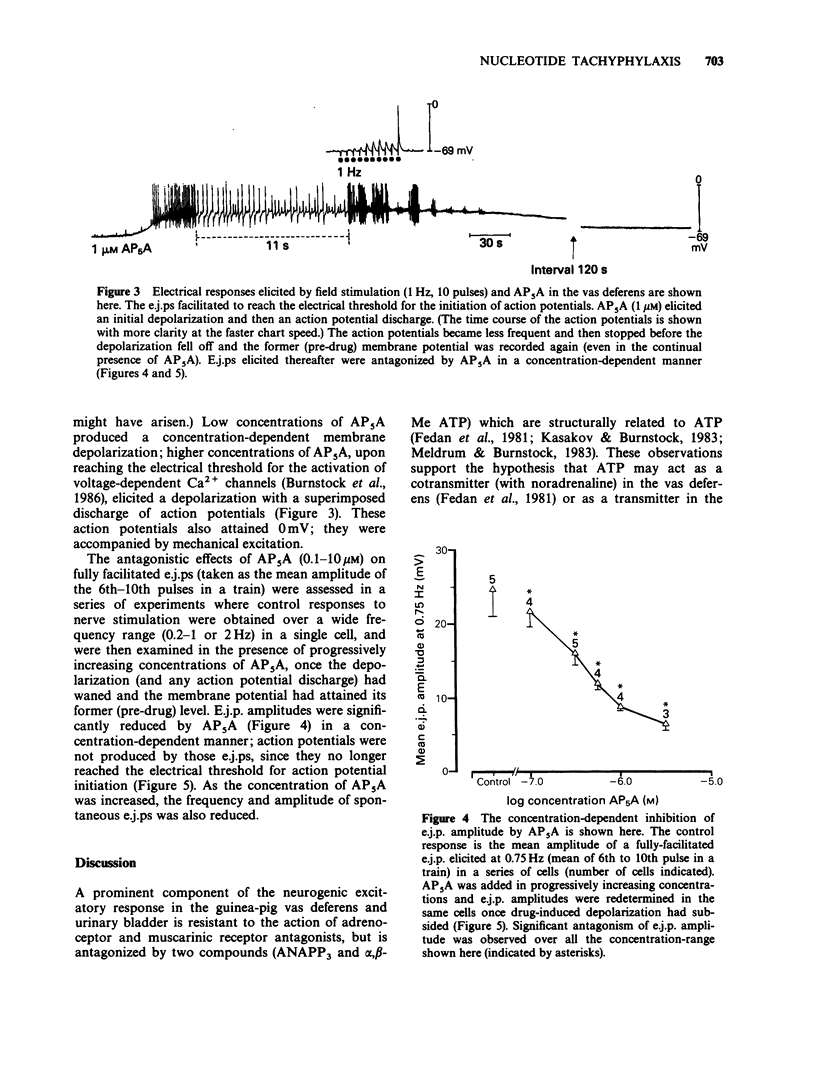
1. The agonistic and antagonistic effects of two nucleotide analogues, P1,P5-di-(adenosine-5') pentaphosphate (AP5A) and alpha,beta-methylene ATP (alpha,beta-Me ATP), have been compared in the guinea-pig isolated vas deferens. 2. In organ bath studies, both AP5A and alpha,beta-Me ATP were approximately 100 times more potent than ATP in producing phasic contractions of the vas deferens smooth muscle. Repeated additions of either agonist (1-10 microM) produced desensitization to a subsequent addition of the test substance. AP5A and alpha,beta-Me ATP were approximately equipotent in the production of desensitization. 3. After desensitization had been produced in the vas deferens by AP5A or alpha,beta-Me ATP, excitatory responses elicited by ATP (100-150 microM) and nonadrenergic field stimulation (2-20 Hz) were blocked, whereas those elicited by carbachol (1-10 microM) were augmented. 4. Intracellular electrical recordings demonstrated that AP5A and alpha,beta-Me ATP produced similar effects on membrane activity of the vas deferens. Concentration-dependent depolarizations alone were produced by both substances until the voltage threshold for action potential discharge was attained; thereafter, action potential discharges were superimposed on the depolarization and an accompanying phasic contraction was recorded. Upon restoration of the membrane potential to its control value (5-10 min after addition of either AP5A or alpha,beta-Me ATP), excitatory junction potentials (e.j.ps) elicited by field stimulation (up to 3 Hz) and spontaneous e.j.ps were reduced by AP5A (greater than 0.1 microM) in a concentration-dependent manner (as previously described for alpha,beta-Me ATP).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allcorn R. J., Cunnane T. C., Kirkpatrick K. Actions of alpha, beta-methylene ATP and 6-hydroxydopamine on sympathetic neurotransmission in the vas deferens of the guinea-pig, rat and mouse: support for cotransmission. Br J Pharmacol. 1986 Dec;89(4):647–659. doi: 10.1111/j.1476-5381.1986.tb11169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambache N., Zar M. A. Evidence against adrenergic motor transmission in the guinea-pig vas deferens. J Physiol. 1971 Jul;216(2):359–389. doi: 10.1113/jphysiol.1971.sp009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. The transmission of excitation from autonomic nerve to smooth muscle. J Physiol. 1961 Jan;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Crowe R., Kasakov L. Purinergic innervation of the guinea-pig urinary bladder. Br J Pharmacol. 1978 May;63(1):125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Warland J. J. P2-purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2y-but not the P2x-purinoceptor. Br J Pharmacol. 1987 Feb;90(2):383–391. doi: 10.1111/j.1476-5381.1987.tb08968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedan J. S., Hogaboom G. K., O'Donnell J. P., Colby J., Westfall D. P. Contribution by purines to the neurogenic response of the vas deferens of the guinea pig. Eur J Pharmacol. 1981 Jan 5;69(1):41–53. doi: 10.1016/0014-2999(81)90600-2. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa S. Actions of ATP and alpha, beta-methylene ATP on neuromuscular transmission and smooth muscle membrane of the rabbit and guinea-pig mesenteric arteries. Br J Pharmacol. 1985 Dec;86(4):777–787. doi: 10.1111/j.1476-5381.1985.tb11099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Saville V. L., Burnstock G. The contributions of noradrenaline and ATP to the responses of the rabbit central ear artery to sympathetic nerve stimulation depend on the parameters of stimulation. Eur J Pharmacol. 1986 Apr 2;122(3):291–300. doi: 10.1016/0014-2999(86)90409-7. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K., Burnstock G. Sympathetic nerve-mediated release of ATP from the guinea-pig vas deferens is unaffected by reserpine. Eur J Pharmacol. 1987 Jun 19;138(2):207–214. doi: 10.1016/0014-2999(87)90434-1. [DOI] [PubMed] [Google Scholar]
- Kolodny N. H., Kisteneff E., Redfield C., Rapaport E. Ap4A and Ap5A prefer folded unstacked conformations at pH 4-5, in sharp contrast with Ap2A and Ap3A. FEBS Lett. 1979 Nov 1;107(1):121–124. doi: 10.1016/0014-5793(79)80477-9. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Secemski I. I. P 1 ,P 5 -Di(adenosine-5')pentaphosphate, a potent multisubstrate inhibitor of adenylate kinase. J Biol Chem. 1973 Feb 10;248(3):1121–1123. [PubMed] [Google Scholar]
- McGrath J. C. Adrenergic and 'non-adrenergic' components in the contractile response of the vas deferens to a single indirect stimulus. J Physiol. 1978 Oct;283:23–39. doi: 10.1113/jphysiol.1978.sp012486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum L. A., Burnstock G. Evidence that ATP acts as a co-transmitter with noradrenaline in sympathetic nerves supplying the guinea-pig vas deferens. Eur J Pharmacol. 1983 Aug 19;92(1-2):161–163. doi: 10.1016/0014-2999(83)90126-7. [DOI] [PubMed] [Google Scholar]
- Momsen G. Firefly luciferase reacts with P1, P5-di(adenosine-5'-)pentaphosphate and adenosine-5'-tetraphosphate. Biochem Biophys Res Commun. 1978 Oct 16;84(3):816–822. doi: 10.1016/0006-291x(78)90777-5. [DOI] [PubMed] [Google Scholar]
- Ogilvie A. Determination of diadenosine tetraphosphate (Ap4A) levels in subpicomole quantities by a phosphodiesterase luciferin--luciferase coupled assay: application as a specific assay for diadenosine tetraphosphatase. Anal Biochem. 1981 Aug;115(2):302–307. doi: 10.1016/0003-2697(81)90009-9. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Coade S. B., Cusack N. J. Characterization of ectonucleotidases on vascular smooth-muscle cells. Biochem J. 1985 Sep 1;230(2):503–507. doi: 10.1042/bj2300503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984 Apr 13;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjärne L., Astrand P. Discrete events measure single quanta of adenosine 5'-triphosphate secreted from sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience. 1984 Sep;13(1):21–28. doi: 10.1016/0306-4522(84)90256-2. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Astrand P. Relative pre- and postjunctional roles of noradrenaline and adenosine 5'-triphosphate as neurotransmitters of the sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience. 1985 Mar;14(3):929–946. doi: 10.1016/0306-4522(85)90155-1. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Actions of adenine dinucleotides on the vas deferens, guinea-pig taenia caeci and bladder. Eur J Pharmacol. 1981 Oct 22;75(2-3):93–102. doi: 10.1016/0014-2999(81)90066-2. [DOI] [PubMed] [Google Scholar]
- Swedin G. Studies on neurotransmission mechanisms in the rat and guinea-pig vas deferens. Acta Physiol Scand Suppl. 1971;369:1–34. [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline and adenosine triphosphate as co-transmitters of neurogenic vasoconstriction in rabbit mesenteric artery. J Physiol. 1985 Oct;367:435–455. doi: 10.1113/jphysiol.1985.sp015834. [DOI] [PMC free article] [PubMed] [Google Scholar]


