Abstract
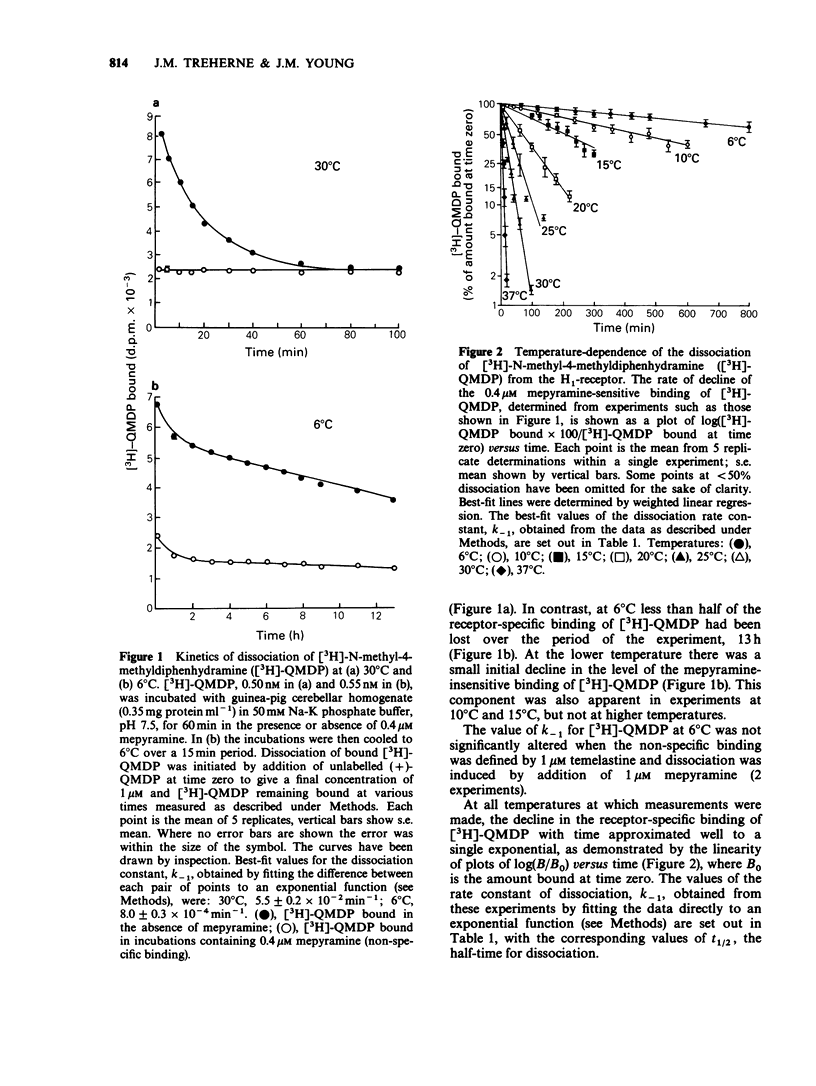
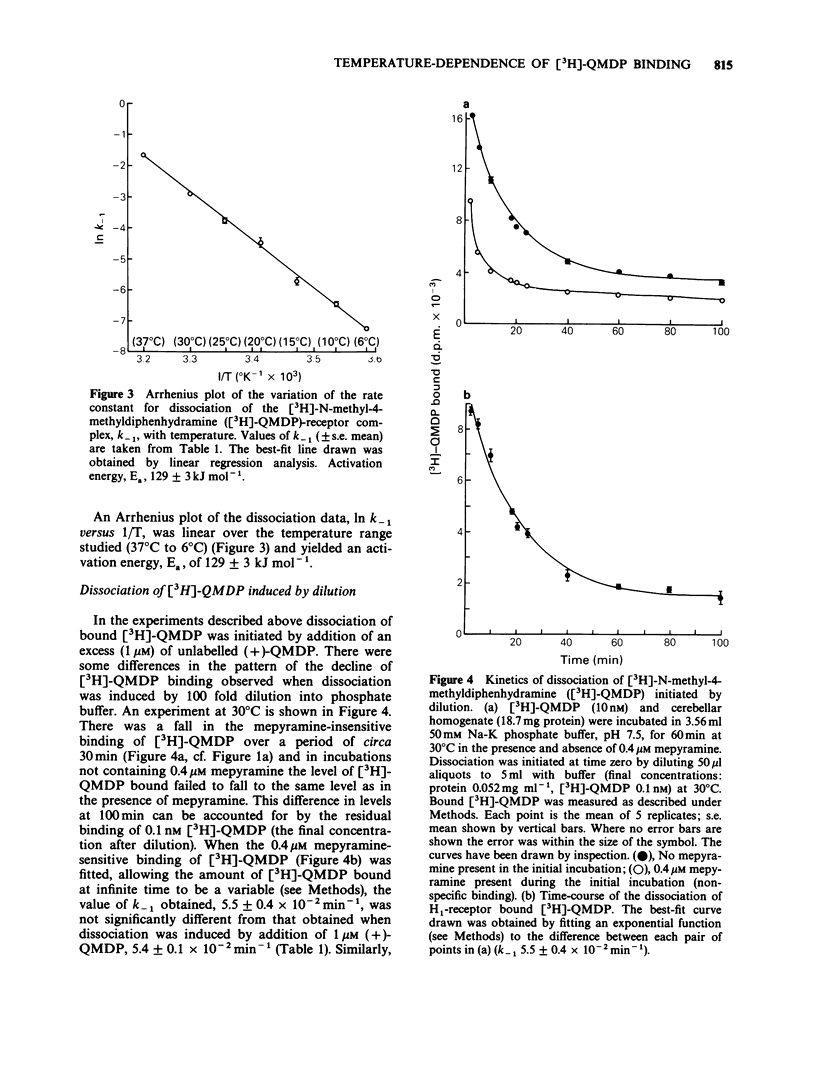
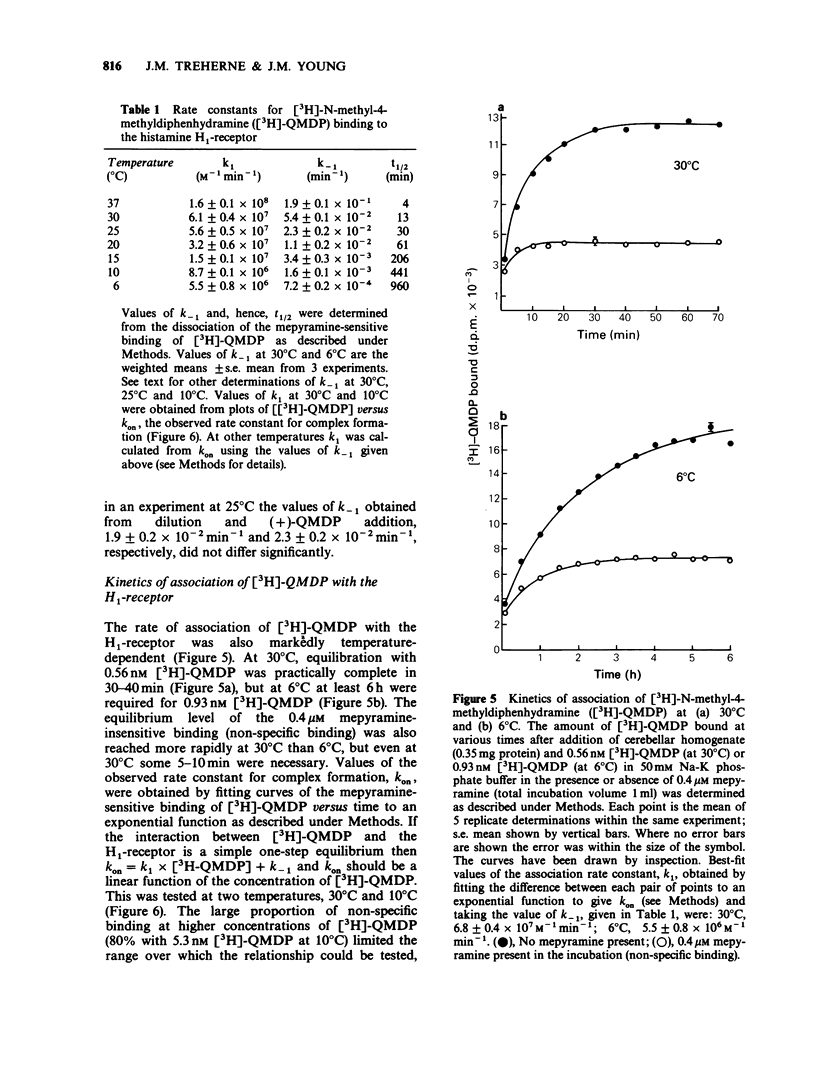
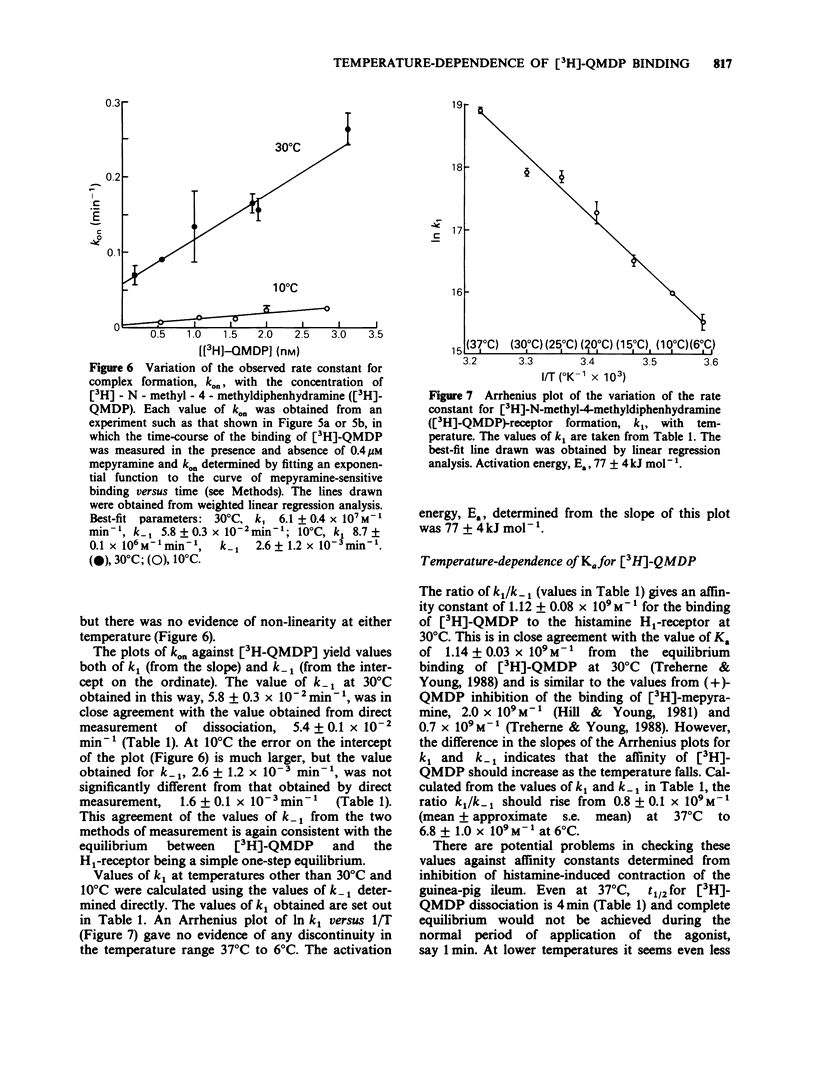
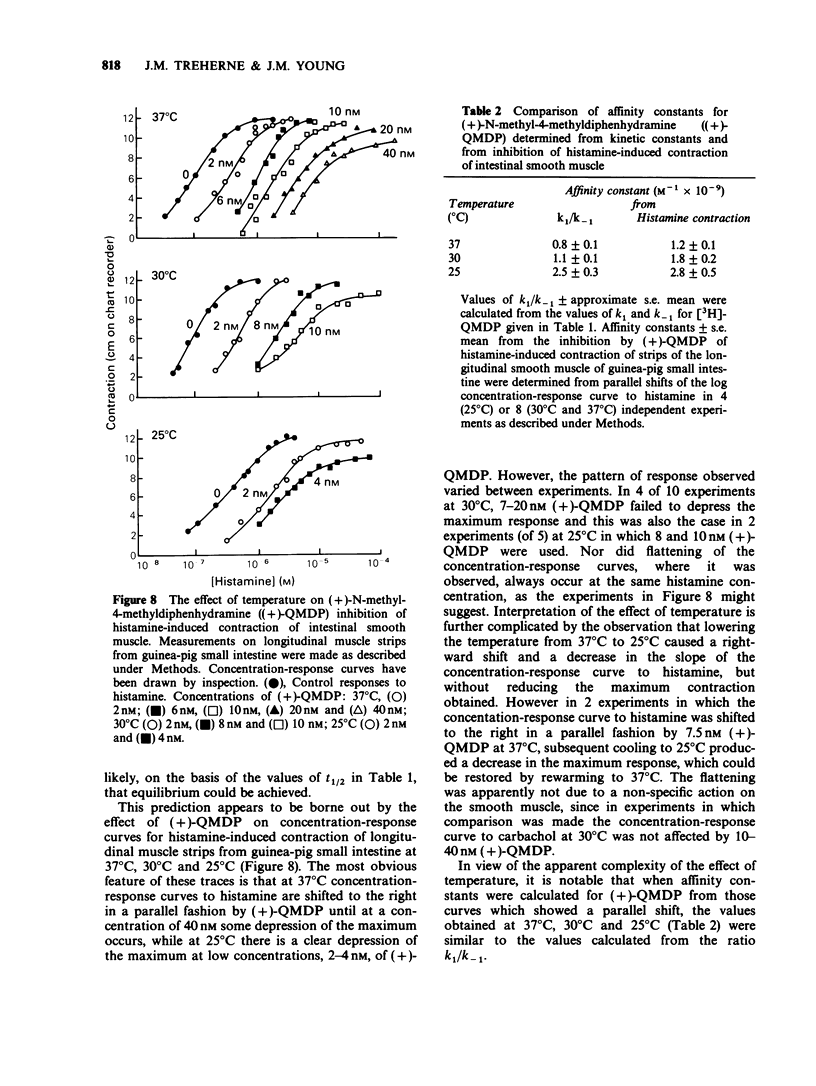
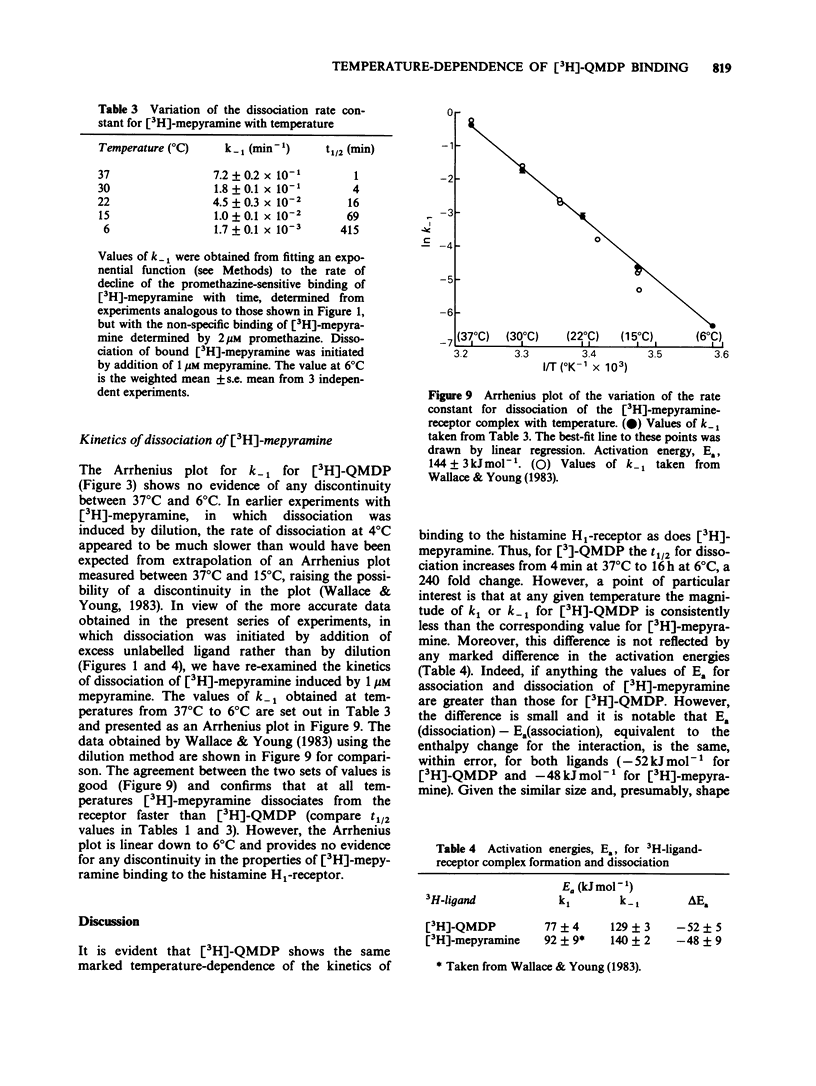
1. The dissociation of [3H]-(+)-N-methyl-4-methyldiphenhydramine ([3H]-QMDP) from the histamine H1-receptor was markedly temperature-dependent. The t1/2 was 4 min at 37 degrees C and 16 h at 6 degrees C. The association rate constant, k1, was also temperature-dependent, but not to the same extent as k-1. 2. Plots of the observed rate constant for [3H]-QMDP-receptor complex formation, kon, versus [3H-QMDP] were linear at both 30 degrees C and 10 degrees C, consistent with the interaction of [3H]-QMDP with the H1-receptor being a simple, one-step equilibrium. 3. The ratio of the kinetic constants, k1/k-1, indicated that the affinity constant of [3H]-QMDP for the H1-receptor should increase with decreasing temperature. Measurement of (+)-QMDP antagonism of the contraction of longitudinal muscle strips from guinea-pig small intestine induced by histamine at 37 degrees C, 30 degrees C and 25 degrees C provided some evidence that the affinity of (+)-QMDP is greater at 25 degrees C than 37 degrees C. However, the flattening of the concentration-response curves for histamine at low concentrations of (+)-QMDP at 30 degrees C and 25 degrees C is consistent with a slow dissociation of the (+)-QMDP-receptor complex and hence an incomplete equilibration with the agonist. 4. Arrhenius plots for k1 and k-1 for [3H]-QMDP were linear between 37 degrees C and 6 degrees C. The activation energies, Ea, for complex formation and dissociation were 77 +/- 4 and 129 +/- 3 kJ mol-1, respectively. 5. An Arrhenius plot for k-1 for the dissociation of [3H]-mepyramine from the H1-receptor was also linear between 37 degrees C and 6 degrees C. The activation energy was 140 +/- 2 kJ mol-1. 6. Activation energies for complex formation with the H1-receptor, Eaf, and complex dissociation, Ead, were similar for [3H]-QMDP and [3H]-mepyramine. The energy difference, Eaf--Ead, equivalent to the enthalpy change, did not differ significantly for the two ligands (-52 and -48 kJ mol-1, respectively). The larger values of k1 and k-1 for [3H]-mepyramine compared to [3H]-QMDP imply the presence of an entropic component in the interaction. 7. The simplest explanation for these observations is that transfer from the aqueous phase into a hydrophobic region is a significant factor in antagonist-H1-receptor interaction. This would be entropically more favourable for [3H]-mepyramine, a tertiary amine, than for [3H]-QMDP, a quaternary amine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook D. A., Krueger C. A., Michalchuk A. Temperature and histamine receptor function--what is really happening? Can J Physiol Pharmacol. 1985 Jun;63(6):751–755. doi: 10.1139/y85-124. [DOI] [PubMed] [Google Scholar]
- Eliard P. H., Rousseau G. G. Thermodynamics of steroid binding to the human glucocorticoid receptor. Biochem J. 1984 Mar 1;218(2):395–404. doi: 10.1042/bj2180395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. J., Young J. M. Characterization of [3H]mepyramine binding to the longitudinal muscle of guinea pig small intestine. Mol Pharmacol. 1981 May;19(3):379–387. [PubMed] [Google Scholar]
- Järv J., Hedlund B., Bartfai T. Isomerization of the muscarinic receptor . antagonist complex. J Biol Chem. 1979 Jul 10;254(13):5595–5598. [PubMed] [Google Scholar]
- Korner M., Bouthenet M. L., Ganellin C. R., Garbarg M., Gros C., Ife R. J., Sales N., Schwartz J. C. [125I]Iodobolpyramine, a highly sensitive probe for histamine H1-receptors in guinea-pig brain. Eur J Pharmacol. 1986 Jan 21;120(2):151–160. doi: 10.1016/0014-2999(86)90535-2. [DOI] [PubMed] [Google Scholar]
- Laduron P. M., Janssen P. F., Gommeren W., Leysen J. E. In vitro and in vivo binding characteristics of a new long-acting histamine H1 antagonist, astemizole. Mol Pharmacol. 1982 Mar;21(2):294–300. [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Schwabe U. Effects of temperature and membrane phase transitions on ligand binding to alpha 2-receptors of human platelets. Mol Pharmacol. 1986 Mar;29(3):228–234. [PubMed] [Google Scholar]
- Plenge P., Mellerup E. T. Imipramine binding site. Temperature dependence of the binding of 3H-labeled imipramine and 3H-labeled paroxetine to human platelet membrane. Biochim Biophys Acta. 1984 Feb 29;770(1):22–28. doi: 10.1016/0005-2736(84)90068-3. [DOI] [PubMed] [Google Scholar]
- Rhodes D. G., Sarmiento J. G., Herbette L. G. Kinetics of binding of membrane-active drugs to receptor sites. Diffusion-limited rates for a membrane bilayer approach of 1,4-dihydropyridine calcium channel antagonists to their active site. Mol Pharmacol. 1985 Jun;27(6):612–623. [PubMed] [Google Scholar]
- Ross P. D., Subramanian S. Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry. 1981 May 26;20(11):3096–3102. doi: 10.1021/bi00514a017. [DOI] [PubMed] [Google Scholar]
- Schreiber G., Henis Y. I., Sokolovsky M. Rate constants of agonist binding to muscarinic receptors in rat brain medulla. Evaluation by competition kinetics. J Biol Chem. 1985 Jul 25;260(15):8795–8802. [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Register R. B., Candelore M. R., Rands E., Dixon R. A. Identification of residues required for ligand binding to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4384–4388. doi: 10.1073/pnas.84.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran V. T., Lebovitz R., Toll L., Snyder S. H. [3H]doxepin interactions with histamine H1-receptors and other sites in guinea pig and rat brain homogenates. Eur J Pharmacol. 1981 Apr 9;70(4):501–509. doi: 10.1016/0014-2999(81)90361-7. [DOI] [PubMed] [Google Scholar]
- Treherne J. M., Young J. M. [3H]-(+)-N-methyl-4-methyldiphenhydramine, a quaternary radioligand for the histamine H1-receptor. Br J Pharmacol. 1988 Jul;94(3):797–810. doi: 10.1111/j.1476-5381.1988.tb11591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. M., Young J. M. Temperature dependence of the binding of [3H]mepyramine and related compounds to the histamine H1 receptor. Mol Pharmacol. 1983 Jan;23(1):60–66. [PubMed] [Google Scholar]


