Abstract
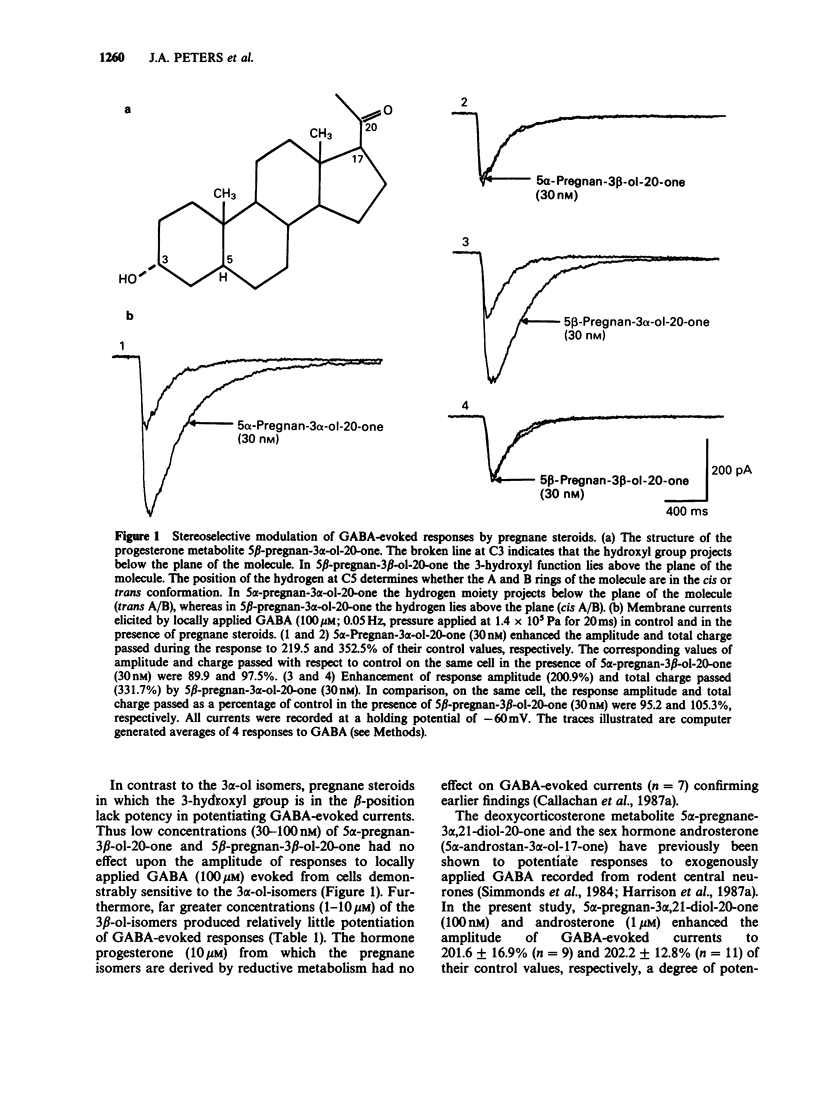
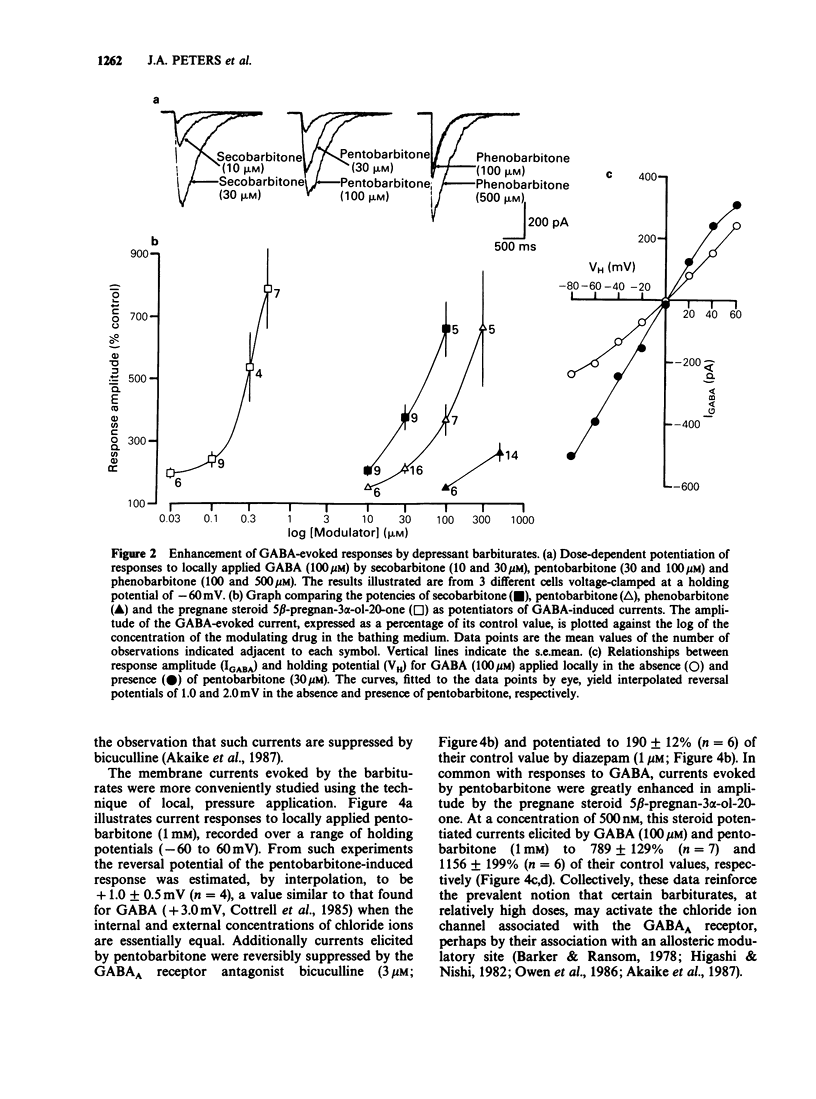
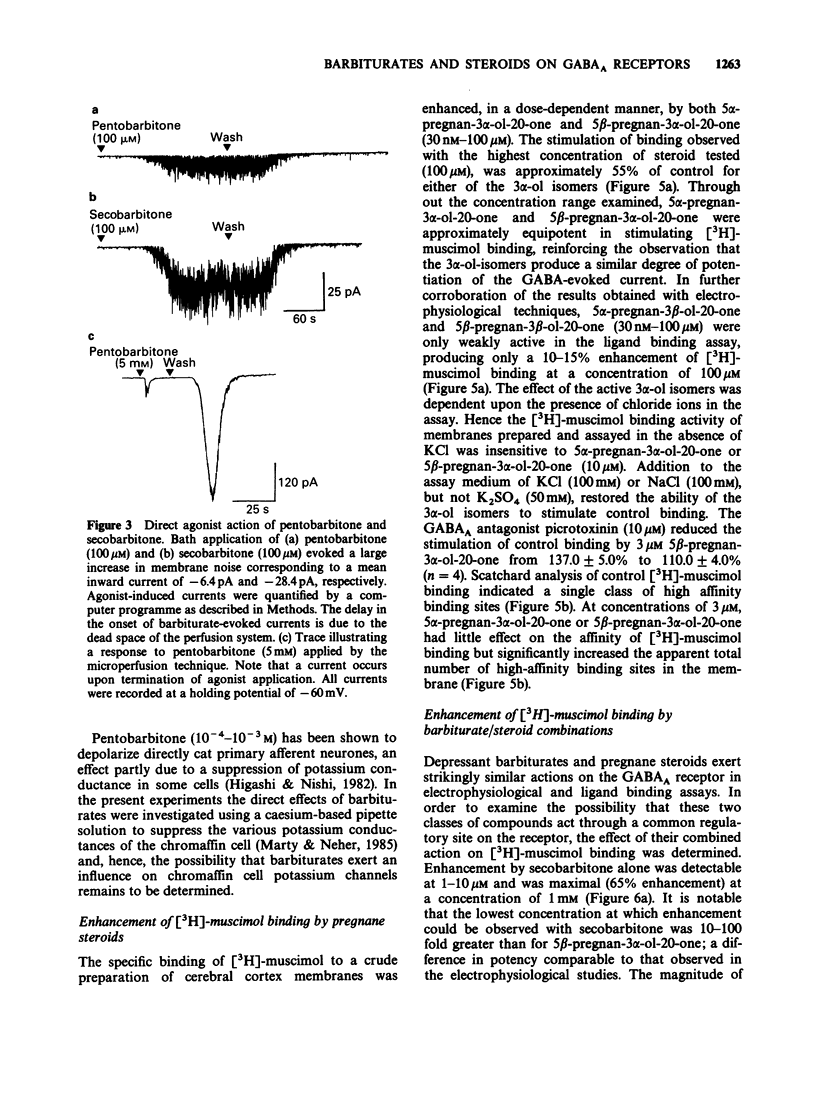
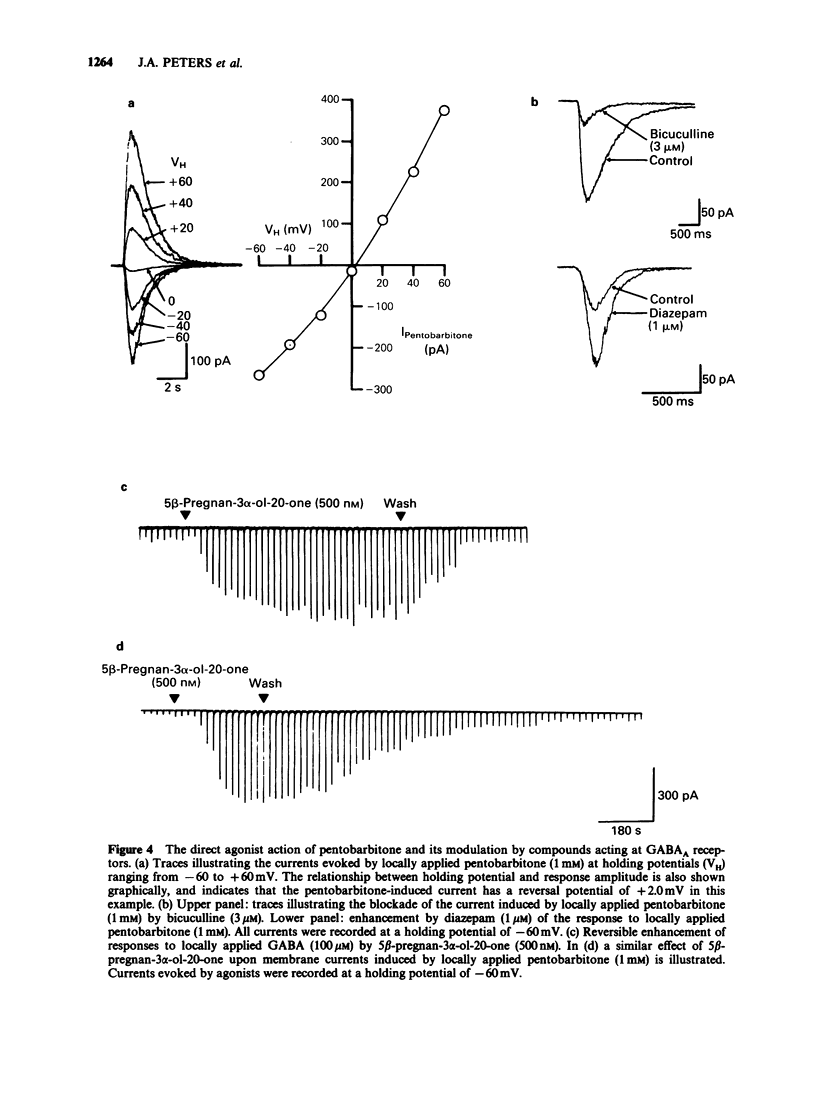
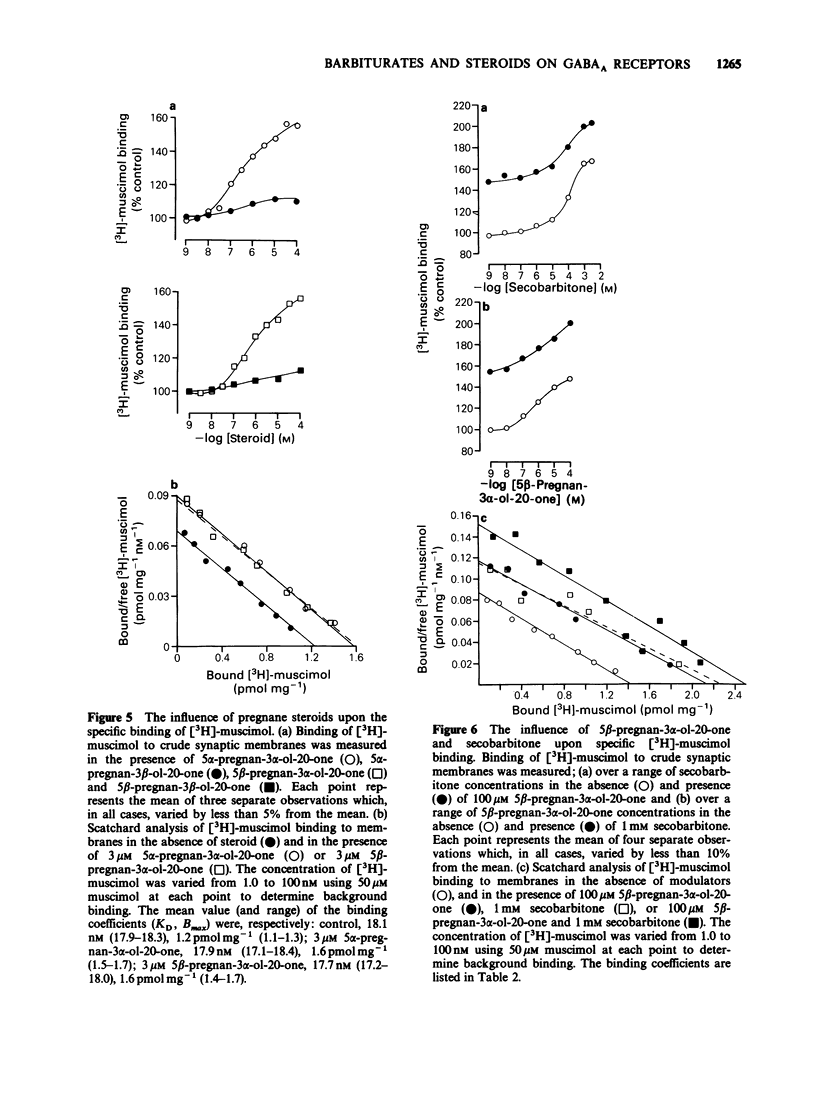
1. The modulation of the gamma-aminobutyric acidA (GABAA) receptor by reduced metabolites of progesterone and deoxycorticosterone has been compared with that produced by depressant barbiturates in: (a) voltage-clamp recordings from bovine enzymatically isolated chromaffin cells in cell culture, and (b) an assay of the specific binding of [3H]-muscimol to a preparation of porcine brain membranes. 2. The progesterone metabolites 5 alpha- and 5 beta-pregnan-3 alpha-ol-20-one (greater than or equal to 30 nM) reversibly and dose-dependently enhanced the amplitude of membrane currents elicited by locally applied GABA (100 microM), and over the concentration range 30 nM-100 microM stimulated the binding of [3H]-muscimol. In contrast, 5 alpha- and 5 beta-pregnan-3 beta-ol-20-one (30 nM-100 microM) had little effect in either assay, indicating a marked stereoselectivity of steroid action. 3. Scatchard analysis of the ligand binding data suggested an apparent increase in the number, rather than the affinity, of detectable [3H]-muscimol binding sites as the principle action of the active steroid isomers. 4. GABA-evoked currents were also potentiated by androsterone (1 microM) and the deoxycorticosterone metabolite 5 alpha-pregnane-3 alpha,21-diol-20-one (100 nM). 5. Secobarbitone (10-100 microM), pentobarbitone (10-300 microM) and phenobarbitone (100-500 microM) reversibly and dose-dependently potentiated the amplitude of GABA-evoked currents in the absence of any change in their reversal potential. 6. At relatively high concentrations (greater than or equal to 30 microM) secobarbitone and pentobarbitone directly elicited a membrane current. It is concluded that such currents result from GABAA receptor-channel activation since they share a common reversal potential with GABA-evoked responses (approximately 0 mV), are reversibly antagonized by bicuculline (3 microM), and potentiated by either diazepam (1 microM) or 5 beta-pregnan-3 alpha-ol-20-one (500 nM). 7. Secobarbitone (1 microM-1 mM) dose-dependently enhanced the binding of [3H]-muscimol. In common with the active steroids, an increase in the apparent number of binding sites was responsible for this effect. 8. A saturating concentration (1 mM) of secobarbitone in the ligand binding assay did not suppress the degree of enhancement of control binding produced by 5 beta-pregnan-3 alpha-ol-20-one (30 nM-100 microM). Similarly the steroid, at a concentration of 100 microM, did not influence the enhancement of [3H]-muscimol binding by secobarbitone (1 microM-1 mM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Maruyama T., Tokutomi N. Kinetic properties of the pentobarbitone-gated chloride current in frog sensory neurones. J Physiol. 1987 Dec;394:85–98. doi: 10.1113/jphysiol.1987.sp016861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks P., Peace C. B. Enzyme inhibition by steroid anaesthetic agents derived from progesterone. Br J Anaesth. 1985 May;57(5):512–514. doi: 10.1093/bja/57.5.512. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Harrison N. L., Lange G. D., Owen D. G. Potentiation of gamma-aminobutyric-acid-activated chloride conductance by a steroid anaesthetic in cultured rat spinal neurones. J Physiol. 1987 May;386:485–501. doi: 10.1113/jphysiol.1987.sp016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Pentobarbitone pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:355–372. doi: 10.1113/jphysiol.1978.sp012388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Clapham D. E. gamma-Aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2168–2172. doi: 10.1073/pnas.82.7.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell G. A., Lambert J. J., Peters J. A. Modulation of GABAA receptor activity by alphaxalone. Br J Pharmacol. 1987 Mar;90(3):491–500. doi: 10.1111/j.1476-5381.1987.tb11198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey M. J., Wardley-Smith B., Wood S. Pressure reversal of alphaxalone/alphadolone and methohexitone in tadpoles: evidence for different molecular sites for general anaesthesia. Br J Pharmacol. 1986 Oct;89(2):299–305. doi: 10.1111/j.1476-5381.1986.tb10260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Majewska M. D., Harrington J. W., Barker J. L. Structure-activity relationships for steroid interaction with the gamma-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987 Apr;241(1):346–353. [PubMed] [Google Scholar]
- Harrison N. L., Simmonds M. A. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984 Dec 10;323(2):287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Vicini S., Barker J. L. A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurosci. 1987 Feb;7(2):604–609. doi: 10.1523/JNEUROSCI.07-02-00604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H., Nishi S. Effect of barbiturates on the GABA receptor of cat primary afferent neurones. J Physiol. 1982 Nov;332:299–314. doi: 10.1113/jphysiol.1982.sp014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane P. E., Biziere K. The effects of general anaesthetics on GABAergic synaptic transmission. Life Sci. 1987 Sep 21;41(12):1437–1448. doi: 10.1016/0024-3205(87)90708-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence D. K., Gill E. W. Structurally specific effects of some steroid anesthetics on spin-labeled liposomes. Mol Pharmacol. 1975 May;11(3):280–286. [PubMed] [Google Scholar]
- Leeb-Lundberg F., Snowman A., Olsen R. W. Barbiturate receptor sites are coupled to benzodiazepine receptors. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7468–7472. doi: 10.1073/pnas.77.12.7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska M. D., Harrison N. L., Schwartz R. D., Barker J. L., Paul S. M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986 May 23;232(4753):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Schwartz R. D. Pregnenolone-sulfate: an endogenous antagonist of the gamma-aminobutyric acid receptor complex in brain? Brain Res. 1987 Feb 24;404(1-2):355–360. doi: 10.1016/0006-8993(87)91394-1. [DOI] [PubMed] [Google Scholar]
- Martin I. L. The benzodiazepines and their receptors: 25 years of progress. Neuropharmacology. 1987 Jul;26(7B):957–970. doi: 10.1016/0028-3908(87)90074-8. [DOI] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W., Snowman A. M. Chloride-dependent enhancement by barbiturates of gamma-aminobutyric acid receptor binding. J Neurosci. 1982 Dec;2(12):1812–1823. doi: 10.1523/JNEUROSCI.02-12-01812.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast U., Brenner O. Modulation of [3H]muscimol binding in rat cerebellar and cerebral cortical membranes by picrotoxin, pentobarbitone, and etomidate. J Neurochem. 1983 Aug;41(2):418–425. doi: 10.1111/j.1471-4159.1983.tb04758.x. [DOI] [PubMed] [Google Scholar]
- Ramanjaneyulu R., Ticku M. K. Binding characteristics and interactions of depressant drugs with [35S]t-butylbicyclophosphorothionate, a ligand that binds to the picrotoxinin site. J Neurochem. 1984 Jan;42(1):221–229. doi: 10.1111/j.1471-4159.1984.tb09721.x. [DOI] [PubMed] [Google Scholar]
- Richards C. D., White A. E. Additive and non-additive effects of mixtures of short-acting intravenous anaesthetic agents and their significance for theories of anaesthesia. Br J Pharmacol. 1981 Sep;74(1):161–170. doi: 10.1111/j.1476-5381.1981.tb09969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory synaptic connections. J Neurophysiol. 1984 Sep;52(3):469–487. doi: 10.1152/jn.1984.52.3.469. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A. Antagonism of flurazepam and other effects of Ro15-1788, PK8165 and Ro5-4864 on the GABA-A receptor complex in rat cuneate nucleus. Eur J Pharmacol. 1985 Oct 29;117(1):51–60. doi: 10.1016/0014-2999(85)90471-6. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A., Turner J. P. Potentiators of responses to activation of gamma-aminobutyric acid (GABAA) receptors. Neuropharmacology. 1987 Jul;26(7B):923–930. doi: 10.1016/0028-3908(87)90071-2. [DOI] [PubMed] [Google Scholar]
- Squires R. F., Casida J. E., Richardson M., Saederup E. [35S]t-butylbicyclophosphorothionate binds with high affinity to brain-specific sites coupled to gamma-aminobutyric acid-A and ion recognition sites. Mol Pharmacol. 1983 Mar;23(2):326–336. [PubMed] [Google Scholar]
- Study R. E., Barker J. L. Diazepam and (--)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan R., Ramanjaneyulu R., Ticku M. K. Enhancement of diazepam and gamma-aminobutyric acid binding by (+)etomidate and pentobarbital. J Neurochem. 1983 Aug;41(2):578–585. doi: 10.1111/j.1471-4159.1983.tb04778.x. [DOI] [PubMed] [Google Scholar]
- Ticku M. K., Rastogi S. K., Thyagarajan R. Separate site(s) of action of optical isomers of 1-methyl-5-phenyl-5-propylbarbituric acid with opposite pharmacological activities at the GABA receptor complex. Eur J Pharmacol. 1985 May 28;112(1):1–9. doi: 10.1016/0014-2999(85)90232-8. [DOI] [PubMed] [Google Scholar]
- Trifiletti R. R., Snowman A. M., Snyder S. H. Barbiturate recognition site on the GABA/benzodiazepine receptor complex is distinct from the picrotoxinin/TBPS recognition site. Eur J Pharmacol. 1984 Nov 13;106(2):441–447. doi: 10.1016/0014-2999(84)90737-4. [DOI] [PubMed] [Google Scholar]
- Whittle S. R., Turner A. J. Differential effects of sedative and anticonvulsant barbiturates on specific [3H]GABA binding to membrane preparations from rat brain cortex. Biochem Pharmacol. 1982 Sep 15;31(18):2891–2895. doi: 10.1016/0006-2952(82)90260-x. [DOI] [PubMed] [Google Scholar]
- Williams M., Risley E. A. Characterization of the binding of [3H]muscimol, a potent gamma-aminobutyric acid agonist, to rat brain synaptosomal membranes using a filtration assay. J Neurochem. 1979 Mar;32(3):713–718. doi: 10.1111/j.1471-4159.1979.tb04553.x. [DOI] [PubMed] [Google Scholar]


