Abstract
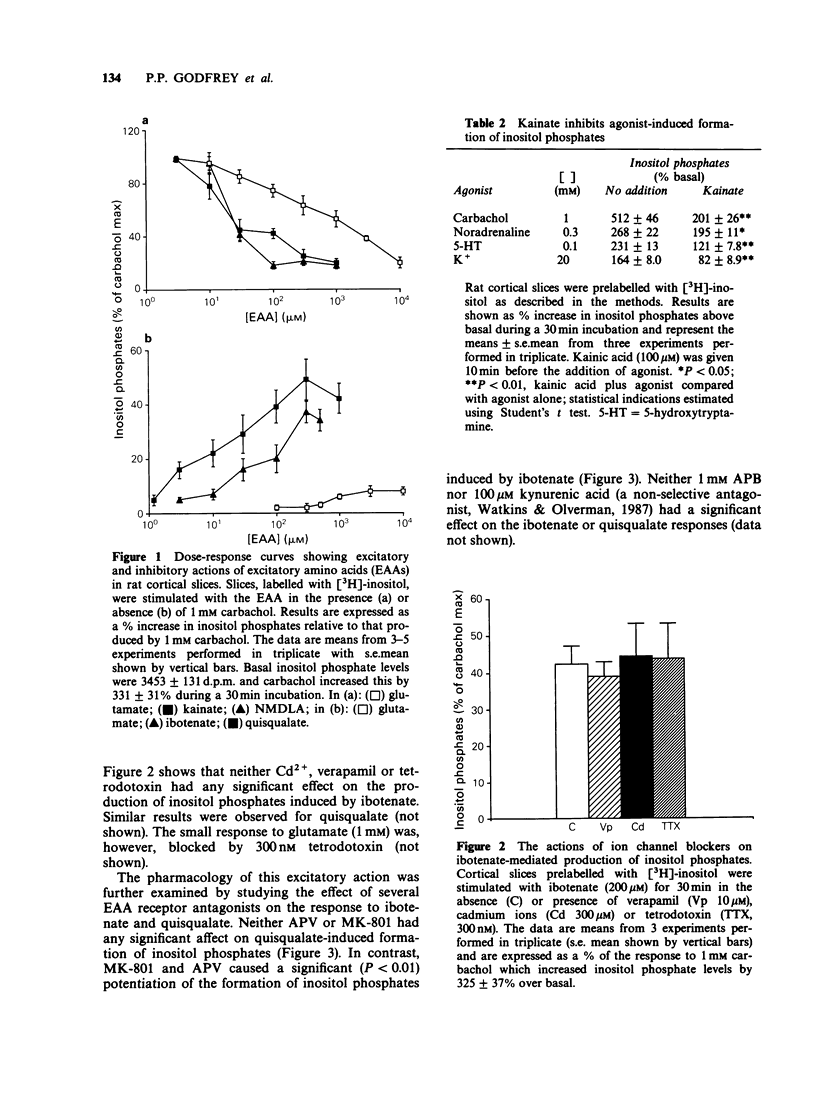
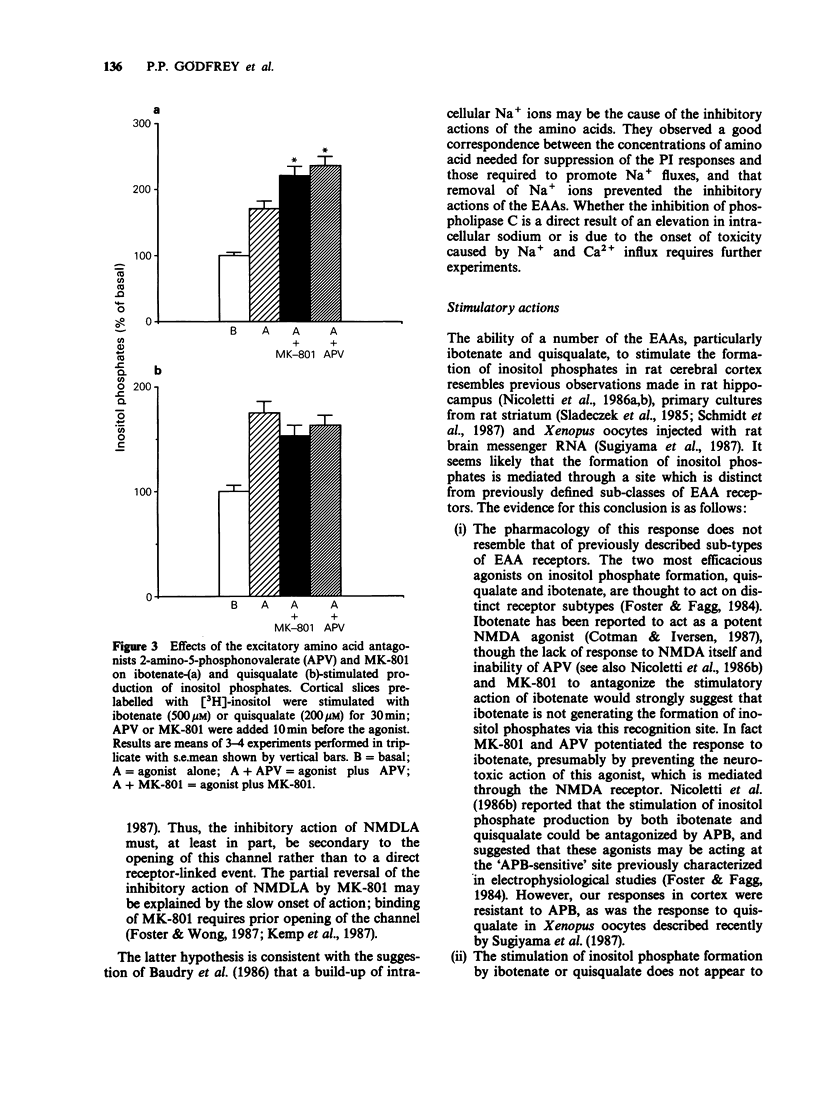
1. The effects of excitatory amino acids on [3H]-inositol phosphate levels have been examined in rat cortical slices under basal conditions or following agonist stimulation. 2. Ibotenate and quisqualate provoked a substantial dose-dependent (EC50, 30 microM and 20 microM respectively) increase in inositol phosphates; these responses were not additive suggesting a common site of action for the two amino acids. The responses to maximally effective concentrations of ibotenate and quisqualate were not blocked by verapamil, tetrodotoxin or Cd2+, indicating that these effects are not indirect. Small, but significant, increases in inositol phosphates were also seen with glutamate and N-methyl-DL-aspartate (NMDLA); kainate and aspartate were ineffective. 3. Each excitatory amino acid tested reduced carbachol (1 mM) stimulated inositol phosphate formation. Kainate (IC50, 20 microM) and NMDLA (IC50, 20 microM) were the most effective inhibitors. Kainate also reduced the responses to noradrenaline, 5-hydroxytryptamine and 20 mM K+. 4. The inhibitory action of NMDLA, but not kainate, could be reversed with the NMDA antagonists, DL-2-amino-5-phosphonovalerate (APV) and MK-801; DL-2-amino-4-phosphonobutyrate (APB) was without effect. Since MK-801 blocks the ion channels associated with the NMDA receptor, it appears that inhibition requires the entry of ions into the cell. 5. APV and MK-801 potentiated the stimulatory response to ibotenate but had no effect on the response to quisqualate. Potentiation was presumably the result of blocking the inhibition by ibotenate mediated through NMDA receptors. 6. In conclusion, excitatory amino acids appear to reduce agonist-mediated inositol phosphate formation in rat cerebral cortex by a non-specific action, possibly including the influx of Na+ ions.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF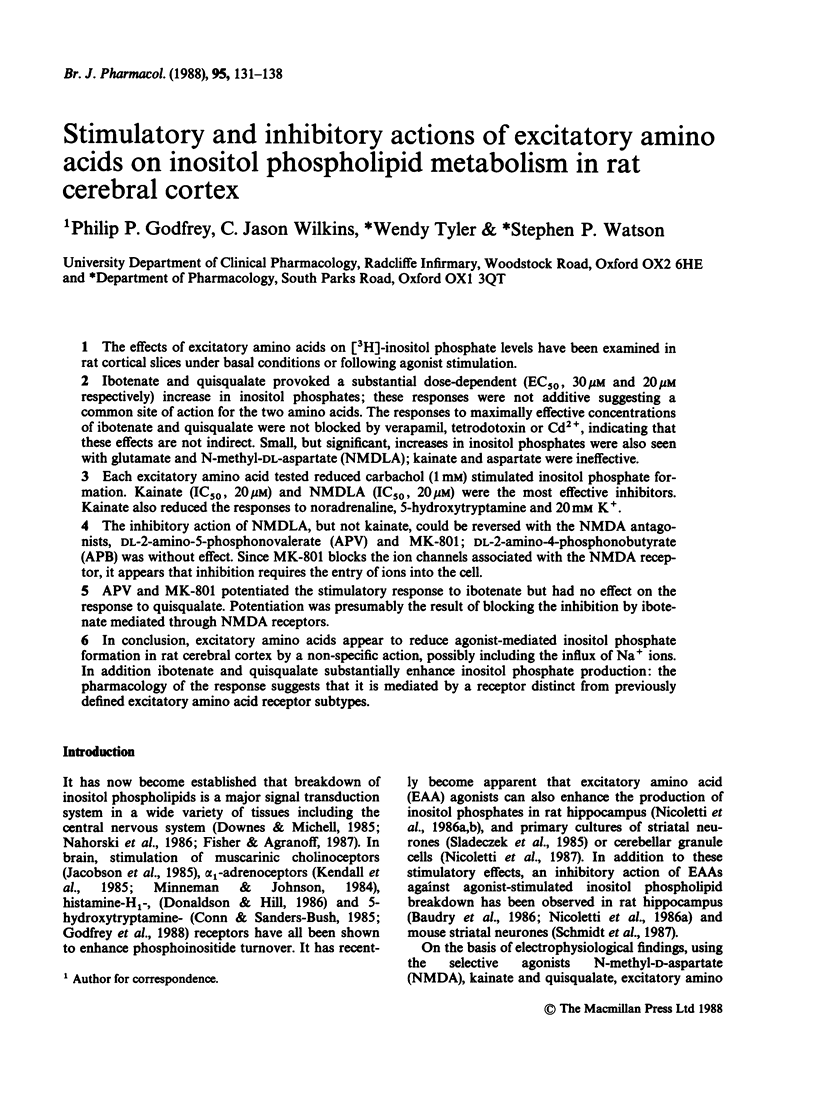





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudry M., Evans J., Lynch G. Excitatory amino acids inhibit stimulation of phosphatidylinositol metabolism by aminergic agonists in hippocampus. Nature. 1986 Jan 23;319(6051):329–331. doi: 10.1038/319329a0. [DOI] [PubMed] [Google Scholar]
- Conn P. J., Sanders-Bush E. Serotonin-stimulated phosphoinositide turnover: mediation by the S2 binding site in rat cerebral cortex but not in subcortical regions. J Pharmacol Exp Ther. 1985 Jul;234(1):195–203. [PubMed] [Google Scholar]
- Donaldson J., Hill S. J. Histamine-induced hydrolysis of polyphosphoinositides in guinea-pig ileum and brain. Eur J Pharmacol. 1986 May 27;124(3):255–265. doi: 10.1016/0014-2999(86)90226-8. [DOI] [PubMed] [Google Scholar]
- Fisher S. K., Agranoff B. W. Receptor activation and inositol lipid hydrolysis in neural tissues. J Neurochem. 1987 Apr;48(4):999–1017. doi: 10.1111/j.1471-4159.1987.tb05618.x. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Wong E. H. The novel anticonvulsant MK-801 binds to the activated state of the N-methyl-D-aspartate receptor in rat brain. Br J Pharmacol. 1987 Jun;91(2):403–409. doi: 10.1111/j.1476-5381.1987.tb10295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. D., Wusteman M., Downes C. P. Muscarinic receptors and hydrolysis of inositol phospholipids in rat cerebral cortex and parotid gland. J Neurochem. 1985 Feb;44(2):465–472. doi: 10.1111/j.1471-4159.1985.tb05437.x. [DOI] [PubMed] [Google Scholar]
- Kendall D. A., Brown E., Nahorski S. R. Alpha 1-adrenoceptor-mediated inositol phospholipid hydrolysis in rat cerebral cortex: relationship between receptor occupancy and response and effects of denervation. Eur J Pharmacol. 1985 Aug 7;114(1):41–52. doi: 10.1016/0014-2999(85)90518-7. [DOI] [PubMed] [Google Scholar]
- Kendall D. A., Nahorski S. R. Dihydropyridine calcium channel activators and antagonists influence depolarization-evoked inositol phospholipid hydrolysis in brain. Eur J Pharmacol. 1985 Sep 10;115(1):31–36. doi: 10.1016/0014-2999(85)90580-1. [DOI] [PubMed] [Google Scholar]
- Minneman K. P., Johnson R. D. Characterization of alpha-1 adrenergic receptors linked to [3H]inositol metabolism in rat cerebral cortex. J Pharmacol Exp Ther. 1984 Aug;230(2):317–323. [PubMed] [Google Scholar]
- Nahorski S. R., Kendall D. A., Batty I. Receptors and phosphoinositide metabolism in the central nervous system. Biochem Pharmacol. 1986 Aug 1;35(15):2447–2453. doi: 10.1016/0006-2952(86)90038-9. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Iadarola M. J., Wroblewski J. T., Costa E. Excitatory amino acid recognition sites coupled with inositol phospholipid metabolism: developmental changes and interaction with alpha 1-adrenoceptors. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1931–1935. doi: 10.1073/pnas.83.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Wroblewski J. T., Costa E. Magnesium ions inhibit the stimulation of inositol phospholipid hydrolysis by endogenous excitatory amino acids in primary cultures of cerebellar granule cells. J Neurochem. 1987 Mar;48(3):967–973. doi: 10.1111/j.1471-4159.1987.tb05611.x. [DOI] [PubMed] [Google Scholar]
- Schmidt B. H., Weiss S., Sebben M., Kemp D. E., Bockaert J., Sladeczek F. Dual action of excitatory amino acids on the metabolism of inositol phosphates in striatal neurons. Mol Pharmacol. 1987 Sep;32(3):364–368. [PubMed] [Google Scholar]
- Sladeczek F., Pin J. P., Récasens M., Bockaert J., Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985 Oct 24;317(6039):717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]


