Abstract
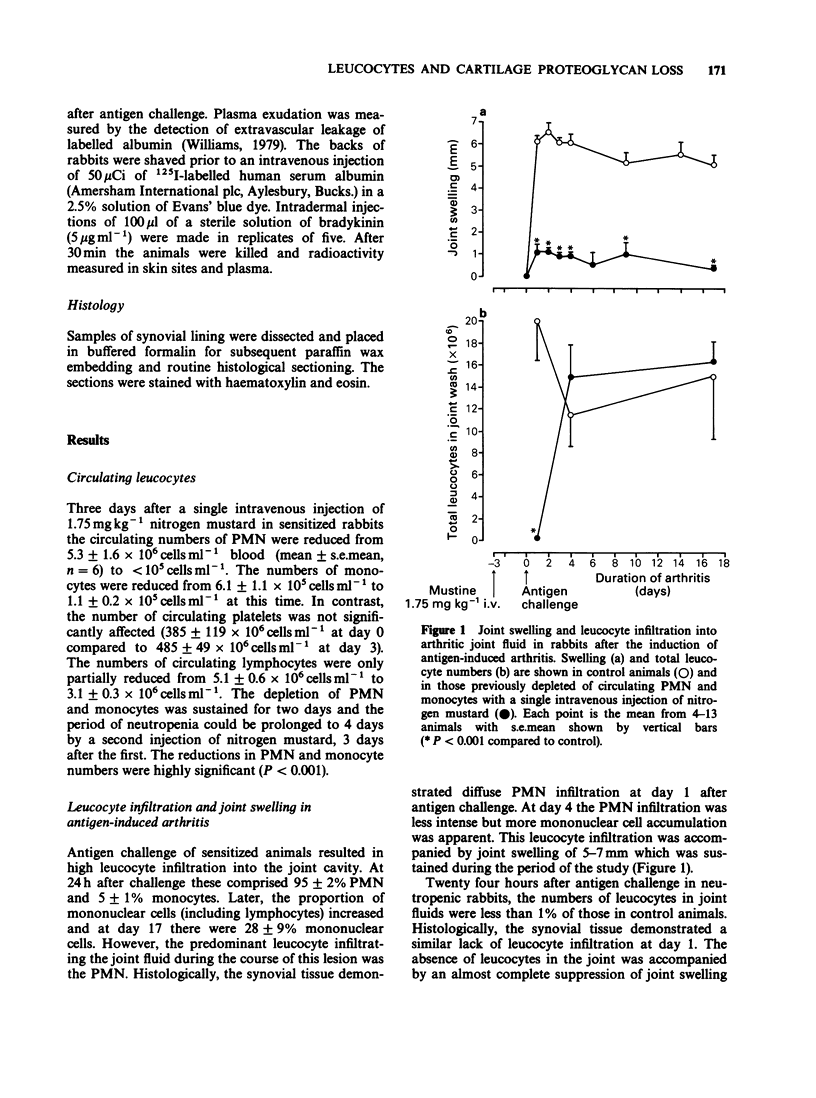
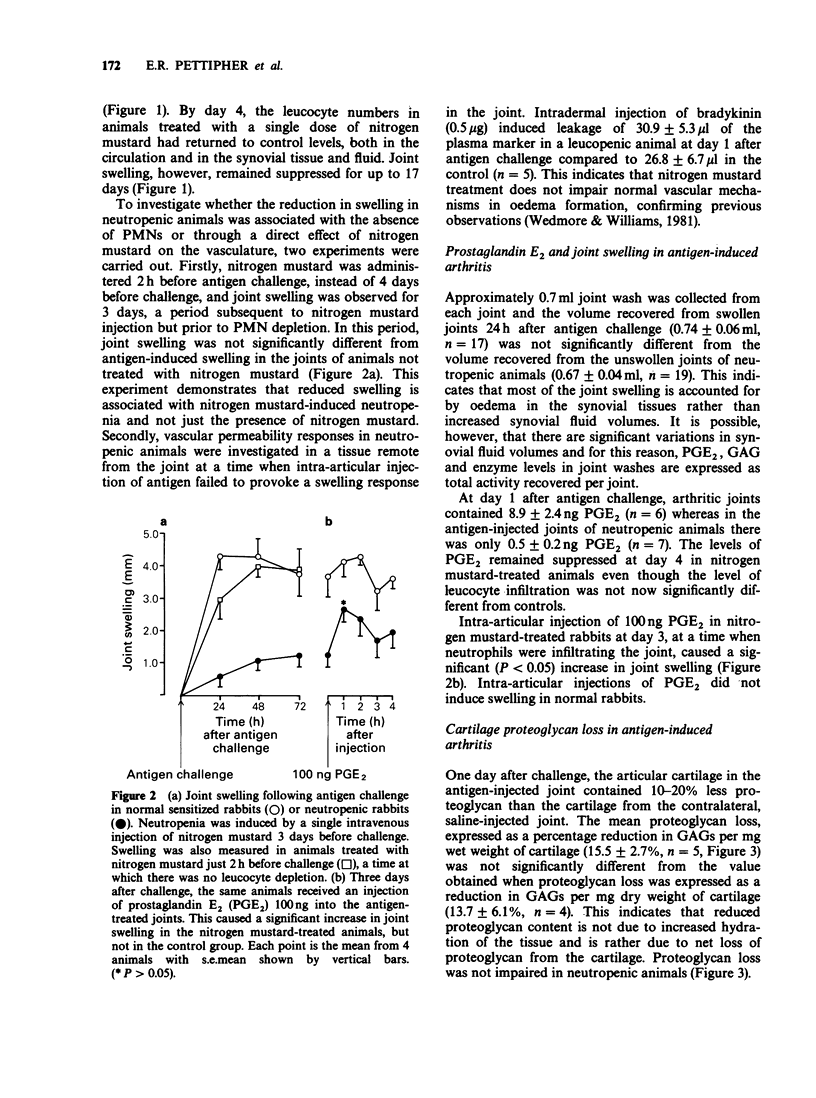
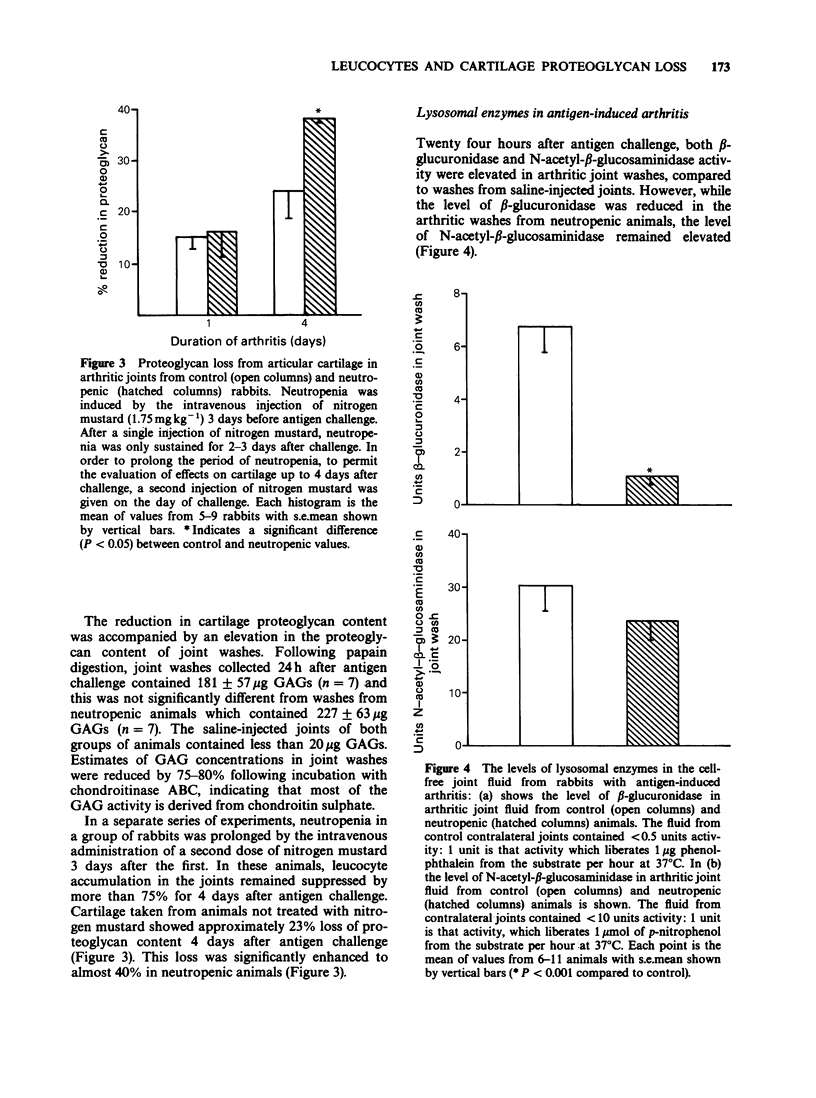
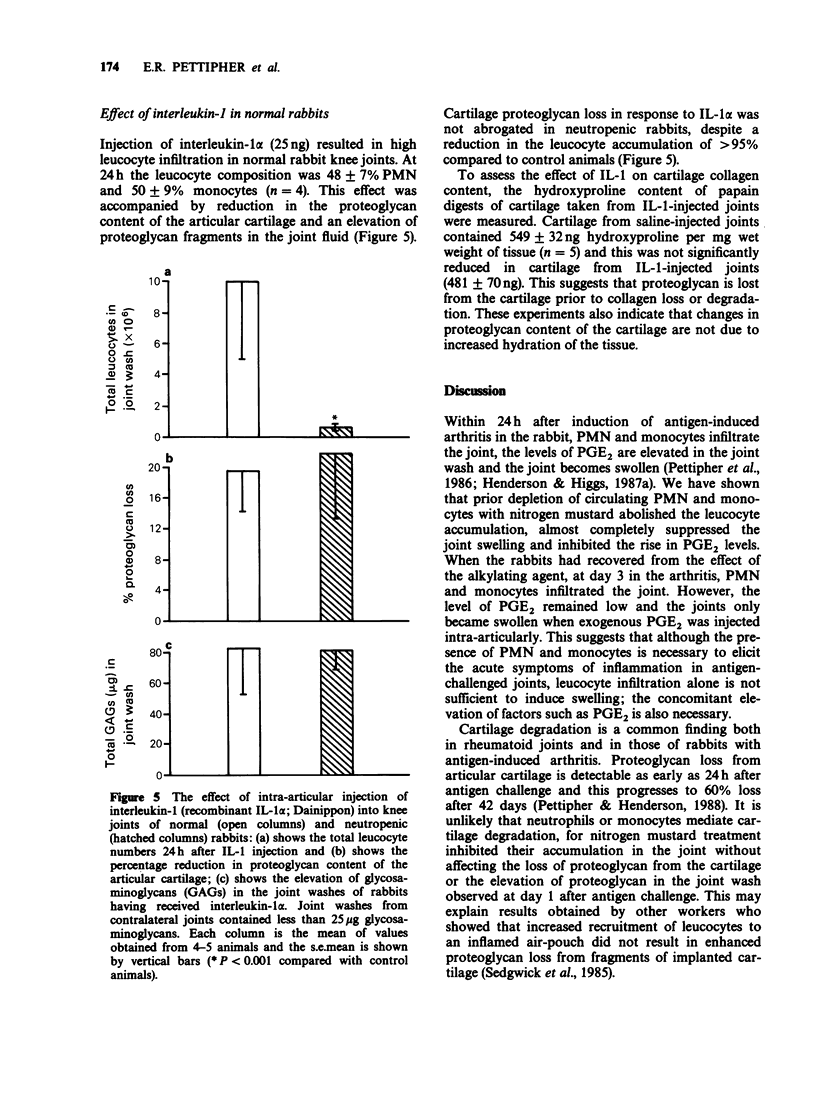
1. The relationship between phagocytic leucocyte infiltration and cartilage degradation in immune arthritis has been investigated in groups of normal and neutropenic rabbits. 2. Injection of antigen into the knee joints of sensitized control animals induced joint swelling, prostaglandin E2 (PGE2) synthesis, leucocyte accumulation and proteoglycan loss from articular cartilage. 3. Intravenous injection of nitrogen mustard caused a selective depletion of circulating neutrophils and monocytes with little or no effect on platelets or lymphocytes. In neutropenic animals challenged with antigen, there was virtually no joint swelling, PGE2 synthesis or leucocyte infiltration but cartilage proteoglycan loss was unchanged after 1 day and increased by day 4 compared to control animals. 4. The numbers of circulating leucocytes returned to normal 3-4 days after nitrogen mustard treatment and leucocyte infiltration occurred in antigen-challenged joints but this was not accompanied by joint swelling. Subsequent intra-articular injection of PGE2 did, however, cause swelling. 5. Lysosomal enzyme levels in arthritic joint fluids were measured. The levels of beta-glucuronidase, which is released by activated phagocytes, were decreased in neutropenic animals but the levels of N-acetyl-beta-glucosaminidase, which is a marker of tissue damage, were not changed by neutrophil depletion. 6. Intra-articular injections of the cytokine interleukin-1 (IL-1) induced a pattern of leucocyte infiltration and cartilage proteoglycan loss similar to that seen in immune arthritis. In neutropenic animals, IL-1 did not cause significant accumulation of leucocytes in the joint but the loss of proteoglycan from cartilage was unimpaired.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUMONDE D. C., GLYNN L. E. The production of arthritis in rabbits by an immunological reaction to fibrin. Br J Exp Pathol. 1962 Aug;43:373–383. [PMC free article] [PubMed] [Google Scholar]
- Farndale R. W., Buttle D. J., Barrett A. J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986 Sep 4;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Fell H. B., Jubb R. W. The effect of synovial tissue on the breakdown of articular cartilage in organ culture. Arthritis Rheum. 1977 Sep-Oct;20(7):1359–1371. doi: 10.1002/art.1780200710. [DOI] [PubMed] [Google Scholar]
- Goto M., Sasano M., Yamanaka H., Miyasaka N., Kamatani N., Inoue K., Nishioka K., Miyamoto T. Spontaneous production of an interleukin 1-like factor by cloned rheumatoid synovial cells in long-term culture. J Clin Invest. 1987 Sep;80(3):786–796. doi: 10.1172/JCI113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B., Glynn L. E. Metabolic alterations in the synoviocytes in chronically inflamed knee joints in immune arthritis in the rabbit: comparison with rheumatoid arthritis. Br J Exp Pathol. 1981 Feb;62(1):27–33. [PMC free article] [PubMed] [Google Scholar]
- Henderson B. Increase in the activity of lysosomal acid hydrolases in the chondrocytes of arthritic joints of rabbits with experimental allergic arthritis. Histochem J. 1984 Mar;16(3):287–293. doi: 10.1007/BF01003612. [DOI] [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- Muirden K. D. Lysosomal enzymes in synovial membrane in rheumatoid arthritis. Relationship to joint damage. Ann Rheum Dis. 1972 Jul;31(4):265–271. doi: 10.1136/ard.31.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oronsky A. L., Perper R. J. Connective tissue-degrading enzymes of human leukocytes. Ann N Y Acad Sci. 1975 Jun 13;256:233–253. doi: 10.1111/j.1749-6632.1975.tb36050.x. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Henderson B. The relationship between cell-mediated immunity and cartilage degradation in antigen-induced arthritis in the rabbit. Br J Exp Pathol. 1988 Feb;69(1):113–122. [PMC free article] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J., Pilsworth L. M., Sarsfield S. J., Gavrilovic J., Heath J. K. Pig catabolin is a form of interleukin 1. Cartilage and bone resorb, fibroblasts make prostaglandin and collagenase, and thymocyte proliferation is augmented in response to one protein. Biochem J. 1984 Dec 1;224(2):461–466. doi: 10.1042/bj2240461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon J. A. A radioimmunoassay for 6-keto-prostaglandin F1alpha. Prostaglandins. 1978 Mar;15(3):383–397. doi: 10.1016/0090-6980(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Sedgwick A. D., Moore A. R., Al-Duaij A. Y., Edwards J. C., Willoughby D. A. Studies into the influence of carrageenan-induced inflammation on articular cartilage degradation using implantation into air pouches. Br J Exp Pathol. 1985 Aug;66(4):445–453. [PMC free article] [PubMed] [Google Scholar]
- Stern D. M., Bank I., Nawroth P. P., Cassimeris J., Kisiel W., Fenton J. W., 2nd, Dinarello C., Chess L., Jaffe E. A. Self-regulation of procoagulant events on the endothelial cell surface. J Exp Med. 1985 Oct 1;162(4):1223–1235. doi: 10.1084/jem.162.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Weissmann G. Lysosomal mechanisms of tissue injury in arthritis. N Engl J Med. 1972 Jan 20;286(3):141–147. doi: 10.1056/NEJM197201202860307. [DOI] [PubMed] [Google Scholar]
- Williams T. J. Prostaglandin E2, prostaglandin I2 and the vascular changes of inflammation. Br J Pharmacol. 1979 Mar;65(3):517–524. doi: 10.1111/j.1476-5381.1979.tb07860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright V., Amos R. Do drugs change the course of rheumatoid arthritis? Br Med J. 1980 Apr 5;280(6219):964–966. doi: 10.1136/bmj.280.6219.964-a. [DOI] [PMC free article] [PubMed] [Google Scholar]


