Abstract
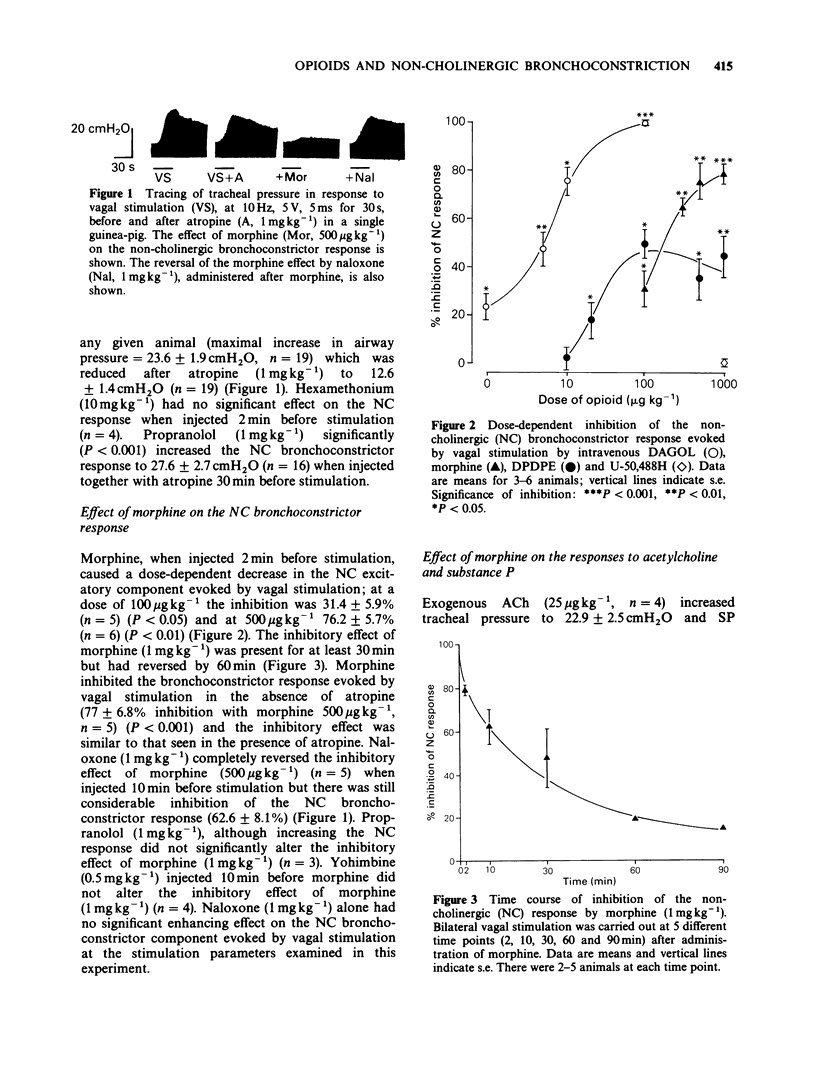
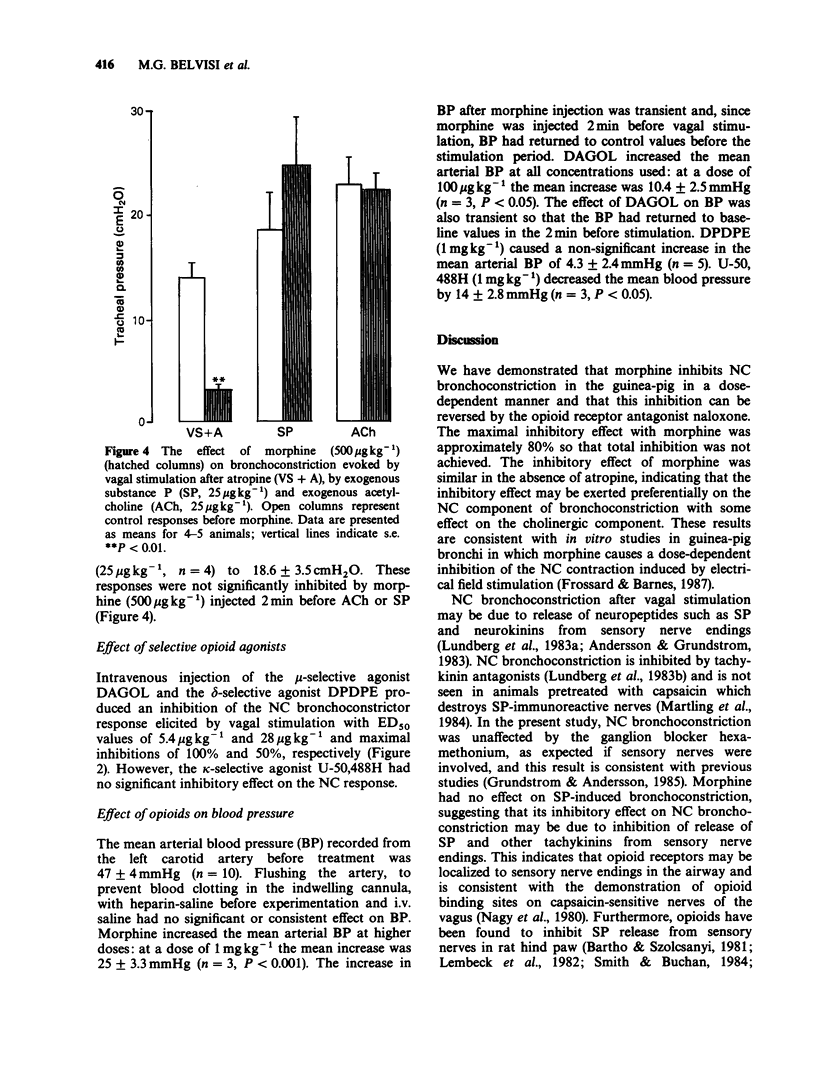
1. Opioid receptors have been demonstrated on sensory fibres in the vagus nerve. Non-cholinergic (NC) neural bronchoconstriction in guinea-pig is due to release of neuropeptides from sensory nerve endings. We have therefore studied the effect of opioids on this NC bronchoconstriction in the anaesthetized guinea-pig. 2. Bilateral vagal stimulation (5 V, 5 ms, 10 Hz) caused reproducible bronchoconstriction in guinea-pigs which was reduced by atropine (1 mg kg-1), but the NC component was unaffected by hexamethonium (10 mg kg-1). 3. NC bronchoconstriction was reduced by morphine in a dose-dependent manner (ED50 = 132 micrograms kg-1 with a maximal inhibition of 79 +/- 2.1% at 1 mg kg-1). Yohimbine (0.5 mg kg-1) did not alter the inhibitory effect of morphine (1 mg kg-1). 4. The inhibitory effect of morphine was completely reversed by naloxone (1 mg kg-1) which had no effect on NC bronchoconstriction. Propranolol (1 mg kg-1) significantly increased the NC bronchoconstrictor response but did not significantly alter the inhibition by morphine. 5. The selective mu-opioid receptor agonist Tyr-(D-Ala)-Gly-(N-Me-Phe)-Glyol (DAGOL) was significantly more potent than morphine with an ED50 of 5.4 micrograms kg-1 and complete inhibition at 100 micrograms kg-1. The delta-agonist Tyr-(D-Pen)-Gly-Phe-(D-Pen) (DPDPE) was less potent than DAGOL with an ED50 of 28 micrograms kg-1 and a maximal inhibition of only 50 +/- 5% at 100 micrograms kg-1.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson R. G., Grundström N. The excitatory non-cholinergic, non-adrenergic nervous system of the guinea-pig airways. Eur J Respir Dis Suppl. 1983;131:141–157. [PubMed] [Google Scholar]
- Atweh S. F., Murrin L. C., Kuhar M. J. Presynaptic localization of opiate receptors in the vagal and accessory optic systems: an autoradiographic study. Neuropharmacology. 1978 Jan;17(1):65–71. doi: 10.1016/0028-3908(78)90175-2. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. Asthma as an axon reflex. Lancet. 1986 Feb 1;1(8475):242–245. doi: 10.1016/s0140-6736(86)90777-4. [DOI] [PubMed] [Google Scholar]
- Barthó L., Szolcsányi J. Opiate agonists inhibit neurogenic plasma extravasation in the rat. Eur J Pharmacol. 1981 Jul 17;73(1):101–104. doi: 10.1016/0014-2999(81)90152-7. [DOI] [PubMed] [Google Scholar]
- Dray A., Nunan L. Selective delta-opioid receptor antagonism by ICI 174,864 in the central nervous system. Peptides. 1984 Sep-Oct;5(5):1015–1016. doi: 10.1016/0196-9781(84)90130-x. [DOI] [PubMed] [Google Scholar]
- Frossard N., Barnes P. J. Mu-opioid receptors modulate non-cholinergic constrictor nerves in guinea-pig airways. Eur J Pharmacol. 1987 Sep 23;141(3):519–522. doi: 10.1016/0014-2999(87)90578-4. [DOI] [PubMed] [Google Scholar]
- Grundström N., Andersson R. G. In vivo demonstration of alpha-2-adrenoceptor-mediated inhibition of the excitatory non-cholinergic neurotransmission in guinea pig airways. Naunyn Schmiedebergs Arch Pharmacol. 1985 Jan;328(3):236–240. doi: 10.1007/BF00515547. [DOI] [PubMed] [Google Scholar]
- Handa B. K., Land A. C., Lord J. A., Morgan B. A., Rance M. J., Smith C. F. Analogues of beta-LPH61-64 possessing selective agonist activity at mu-opiate receptors. Eur J Pharmacol. 1981 Apr 9;70(4):531–540. doi: 10.1016/0014-2999(81)90364-2. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Mosberg H. I., Hurst R., Hruby V. J., Burks T. F., Porreca F. Studies in vitro with ICI 174,864, [D-Pen2, D-Pen5]-enkephalin (DPDPE) and [D-Ala2, NMePhe4, Gly-ol]-enkephalin (DAGO). Neuropeptides. 1985 Feb;5(4-6):383–386. doi: 10.1016/0143-4179(85)90034-4. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Iversen L. L. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature. 1977 Aug 11;268(5620):549–551. doi: 10.1038/268549a0. [DOI] [PubMed] [Google Scholar]
- Konishi S., Tsunoo A., Otsuka M. Enkephalin as a transmitter for presynaptic inhibition in sympathetic ganglia. Nature. 1981 Nov 5;294(5836):80–82. doi: 10.1038/294080a0. [DOI] [PubMed] [Google Scholar]
- Laduron P. M. Axonal transport of opiate receptors in capsaicin-sensitive neurones. Brain Res. 1984 Feb 27;294(1):157–160. doi: 10.1016/0006-8993(84)91322-2. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Donnerer J., Barthó L. Inhibition of neurogenic vasodilation and plasma extravasation by substance P antagonists, somatostatin and [D-Met2, Pro5]enkephalinamide. Eur J Pharmacol. 1982 Nov 19;85(2):171–176. doi: 10.1016/0014-2999(82)90462-9. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Donnerer J. Opioid control of the function of primary afferent substance P fibres. Eur J Pharmacol. 1985 Aug 27;114(3):241–246. doi: 10.1016/0014-2999(85)90365-6. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Brodin E., Saria A. Effects and distribution of vagal capsaicin-sensitive substance P neurons with special reference to the trachea and lungs. Acta Physiol Scand. 1983 Nov;119(3):243–252. doi: 10.1111/j.1748-1716.1983.tb07334.x. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A., Brodin E., Rosell S., Folkers K. A substance P antagonist inhibits vagally induced increase in vascular permeability and bronchial smooth muscle contraction in the guinea pig. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1120–1124. doi: 10.1073/pnas.80.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martling C. R., Saria A., Andersson P., Lundberg J. M. Capsaicin pretreatment inhibits vagal cholinergic and non-cholinergic control of pulmonary mechanics in the guinea pig. Naunyn Schmiedebergs Arch Pharmacol. 1984 Apr;325(4):343–348. doi: 10.1007/BF00504379. [DOI] [PubMed] [Google Scholar]
- Mosberg H. I., Hurst R., Hruby V. J., Gee K., Yamamura H. I., Galligan J. J., Burks T. F. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J. I., Vincent S. R., Staines W. A., Fibiger H. C., Reisine T. D., Yamamura H. I. Neurotoxic action of capsaicin on spinal substance P neurons. Brain Res. 1980 Mar 31;186(2):435–444. doi: 10.1016/0006-8993(80)90987-7. [DOI] [PubMed] [Google Scholar]
- Pasternak G. W. Multiple mu opiate receptors: biochemical and pharmacological evidence for multiplicity. Biochem Pharmacol. 1986 Feb 1;35(3):361–364. doi: 10.1016/0006-2952(86)90205-4. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A., Pasi A., Mehraein P., Herz A. Opiate receptor binding sites in human brain. Brain Res. 1982 Sep 23;248(1):87–96. doi: 10.1016/0006-8993(82)91150-7. [DOI] [PubMed] [Google Scholar]
- Piercey M. F., Lahti R. A., Schroeder L. A., Einspahr F. J., Barsuhn C. U-50488H, a pure kappa receptor agonist with spinal analgesic loci in the mouse. Life Sci. 1982 Sep 20;31(12-13):1197–1200. doi: 10.1016/0024-3205(82)90341-1. [DOI] [PubMed] [Google Scholar]
- Smith T. W., Buchan P. Peripheral opioid receptors located on the rat saphenous nerve. Neuropeptides. 1984 Dec;5(1-3):217–220. doi: 10.1016/0143-4179(84)90066-0. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Wamsley J. K., Zarbin M. A., Kuhar M. J. Opioid receptors undergo axonal flow. Science. 1980 Oct 3;210(4465):76–78. doi: 10.1126/science.6158097. [DOI] [PubMed] [Google Scholar]


