Abstract
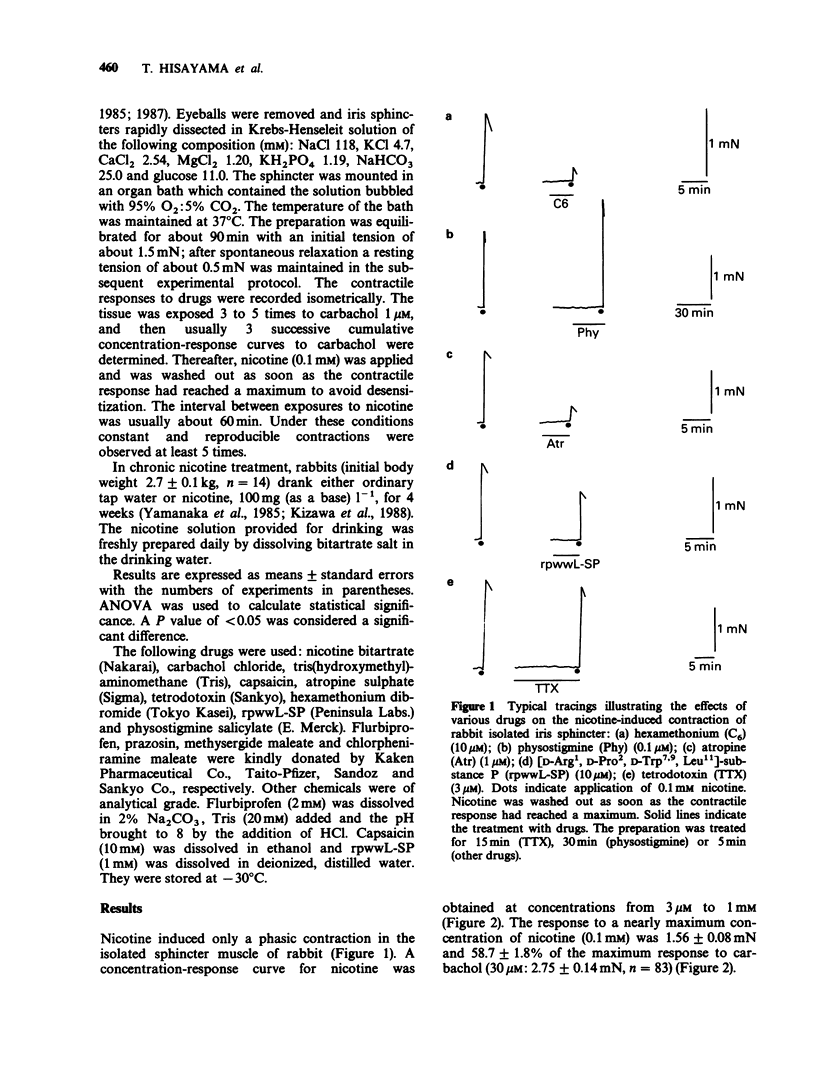
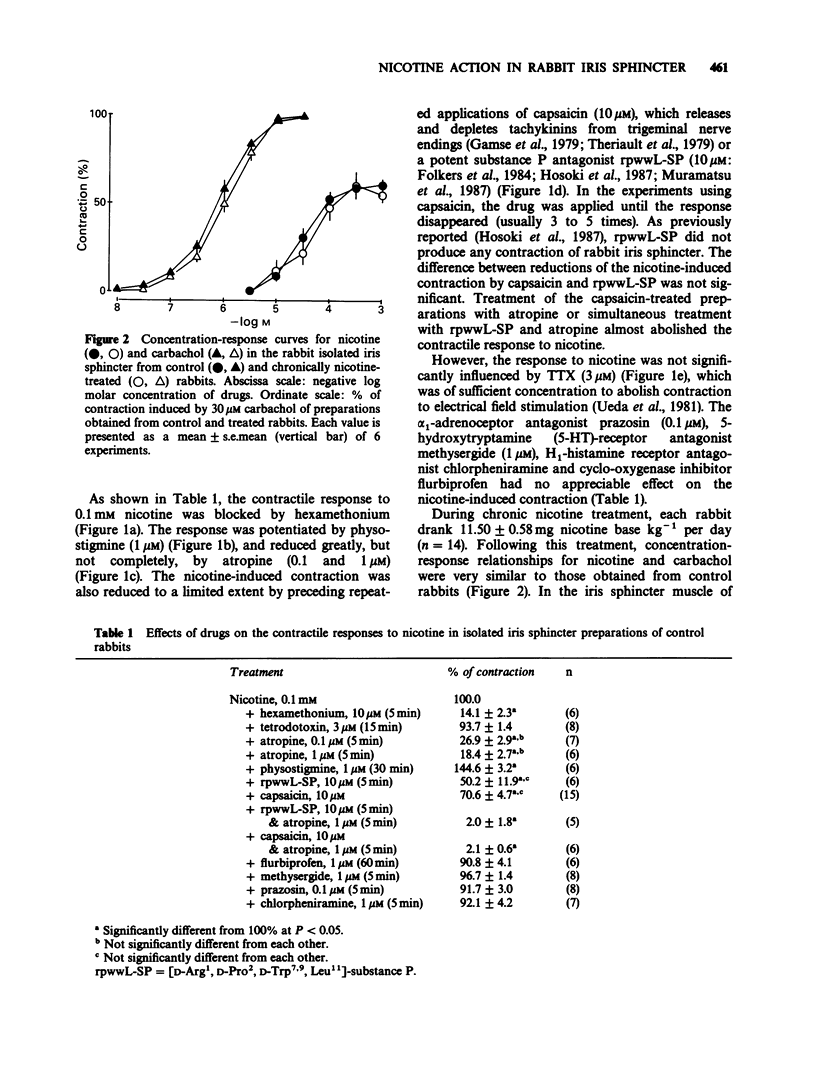
1. Nicotine produced a transient contraction of rabbit isolated iris sphincter muscle, a parasympathetic ganglion-free tissue. The response to nicotine was antagonized by hexamethonium, but was insensitive to tetrodotoxin (TTX). While single treatments with atropine, capsaicin or [D-Arg1, D-Pro2, D-Trp7,9, Leu11]-substance P (rpwwL-SP) partially blocked the response, combined treatment abolished it. 2. Chronic treatment of animals with nicotine added to the drinking water (about 12 mg kg-1 per day) had no effect on the responsiveness to nicotine or the pharmacological properties of nicotine-induced contraction. 3. These results suggest that acetylcholine and tachykinin(s) released via sodium channel-independent mechanisms from nerve terminals of parasympathetic and primary sensory nerves, respectively, are involved in the nicotine-induced contractile response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Folkers K., Håkanson R., Hörig J., Xu J. C., Leander S. Biological evaluation of substance P antagonists. Br J Pharmacol. 1984 Oct;83(2):449–456. doi: 10.1111/j.1476-5381.1984.tb16506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamse R., Molnar A., Lembeck F. Substance P release from spinal cord slices by capsaicin. Life Sci. 1979 Aug 13;25(7):629–636. doi: 10.1016/0024-3205(79)90558-7. [DOI] [PubMed] [Google Scholar]
- Hasegawa N., Imaizumi Y., Watanabe M. Parasympathetic denervation supersensitivity in the rat iris sphincter muscle: an in vitro study. Jpn J Pharmacol. 1987 Feb;43(2):143–151. doi: 10.1254/jjp.43.143. [DOI] [PubMed] [Google Scholar]
- Hosoki R., Hisayama T., Takayanagi I. Pharmacological evidence for the possible coexistence of multiple receptor sites for mammalian tachykinins in rabbit iris sphincter smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1987 Mar;335(3):290–295. doi: 10.1007/BF00172799. [DOI] [PubMed] [Google Scholar]
- Hosoki R., Hisayama T., Takayanagi I. Some characterization of the responses to substance P and other tachykinins in rabbit iris sphincter muscle. Jpn J Pharmacol. 1985 Feb;37(2):159–165. doi: 10.1254/jjp.37.159. [DOI] [PubMed] [Google Scholar]
- Häggendal J., Henning M. Effect of chronically administered nicotine on axonal transport of dopamine-beta-hydroxylase in peripheral adrenergic neurons and on blood pressure and heart rate in the rat. Acta Physiol Scand Suppl. 1980;479:35–38. [PubMed] [Google Scholar]
- Ikushima S., Muramatsu I., Fujiwara M. Effects of 4-aminopyridine on the adrenergic nerve terminals of rabbit arteries. J Pharmacol Exp Ther. 1981 Dec;219(3):792–797. [PubMed] [Google Scholar]
- Ikushima S., Muramatsu I., Fujiwara M. Nicotine-induced response in guinea-pig aorta enhanced by goniopora toxin. J Pharmacol Exp Ther. 1982 Dec;223(3):790–794. [PubMed] [Google Scholar]
- Jayasundar S., Vohra M. M. Mechanism of nicotine-induced release of norepinephrine from adrenergic nerve endings: is generation and propagation of impulses necessary? Arch Int Pharmacodyn Ther. 1978 Apr;232(2):202–210. [PubMed] [Google Scholar]
- Kizawa Y., Takayanagi I. Mechanism of action of nicotine on crab-eating monkey isolated bronchial smooth muscle. Can J Physiol Pharmacol. 1985 Nov;63(11):1471–1473. doi: 10.1139/y85-241. [DOI] [PubMed] [Google Scholar]
- Kizawa Y., Takayanagi I. Possible involvement of substance P immunoreactive nerves in the mediation of nicotine-induced contractile responses in isolated guinea pig bronchus. Eur J Pharmacol. 1985 Jul 31;113(3):319–323. doi: 10.1016/0014-2999(85)90079-2. [DOI] [PubMed] [Google Scholar]
- Kizawa Y., Takayanagi I., Shinkai M., Ohno Y. Pharmacological action of nicotine in the isolated urinary bladder from rabbit: special reference to the chronic nicotine treatment. Gen Pharmacol. 1988;19(2):269–271. doi: 10.1016/0306-3623(88)90074-2. [DOI] [PubMed] [Google Scholar]
- Marks M. J., Burch J. B., Collins A. C. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983 Sep;226(3):817–825. [PubMed] [Google Scholar]
- Muramatsu I., Nakanishi S., Fujiwara M. Comparison of the responses to the sensory neuropeptides, substance P, neurokinin A, neurokinin B and calcitonin gene-related peptide and to trigeminal nerve stimulation in the iris sphincter muscle of the rabbit. Jpn J Pharmacol. 1987 May;44(1):85–92. doi: 10.1254/jjp.44.85. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Nawa H., Kotani H., Nakanishi S. Tissue-specific generation of two preprotachykinin mRNAs from one gene by alternative RNA splicing. Nature. 1984 Dec 20;312(5996):729–734. doi: 10.1038/312729a0. [DOI] [PubMed] [Google Scholar]
- Schaeppi U. Postganglionic nature of parasympathetic innervation of pig iris sphincter. Am J Physiol. 1966 Jan;210(1):91–94. doi: 10.1152/ajplegacy.1966.210.1.91. [DOI] [PubMed] [Google Scholar]
- Su C., Bevan J. A. Blockade of the nicotine-induced norepinephrine release by cocaine, phenoxybenzamine and desipramine. J Pharmacol Exp Ther. 1970 Nov;175(2):533–540. [PubMed] [Google Scholar]
- Szüts T., Olsson S., Lindquist N. G., Ullberg S., Pilotti A., Enzell C. Long-term fate of [14C]nicotine in the mouse: retention in the bronchi, melanin-containing tissues and urinary bladder wall. Toxicology. 1978 Jul;10(3):207–220. doi: 10.1016/0300-483x(78)90072-0. [DOI] [PubMed] [Google Scholar]
- Takayanagi I., Kizawa Y., Hiruta T. Tetrodotoxin-resistant response to nicotine in rabbit bronchial preparation. Eur J Pharmacol. 1984 Sep 17;104(3-4):351–356. doi: 10.1016/0014-2999(84)90412-6. [DOI] [PubMed] [Google Scholar]
- Takayanagi I., Kizawa Y., Toyoda T., Furukawa A. Characterization of nicotine-induced contraction in the canine bronchus. Comp Biochem Physiol C. 1988;89(1):11–13. doi: 10.1016/0742-8413(88)90139-9. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Fujiwara M., Masuo Y., Kanazawa I. Levels of neurokinin A, neurokinin B and substance P in rabbit iris sphincter muscle. Jpn J Pharmacol. 1986 Dec;42(4):590–593. doi: 10.1254/jjp.42.590. [DOI] [PubMed] [Google Scholar]
- Theriault E., Otsuka M., Jessell T. Capsaicin-evoked release of substance P from primary sensory neurons. Brain Res. 1979 Jul 6;170(1):209–213. doi: 10.1016/0006-8993(79)90957-0. [DOI] [PubMed] [Google Scholar]
- Ueda N., Muramatsu I., Hayashi H., Fujiwara M. Trigeminal nerve: the possible origin of substance p-nergic response in isolated rabbit iris sphincter muscle. Life Sci. 1982 Jul 26;31(4):369–375. doi: 10.1016/0024-3205(82)90417-9. [DOI] [PubMed] [Google Scholar]
- Ueda N., Muramatsu I., Sakakibara Y., Fujiwara M. Noncholinergic, nonadrenergic contraction and substance P in rabbit iris sphincter muscle. Jpn J Pharmacol. 1981 Dec;31(6):1071–1079. doi: 10.1254/jjp.31.1071. [DOI] [PubMed] [Google Scholar]
- Yamanaka K., Oshita M., Muramatsu I. Alteration of alpha and muscarinic receptors in rat brain and heart following chronic nicotine treatment. Brain Res. 1985 Dec 2;348(2):241–248. doi: 10.1016/0006-8993(85)90442-1. [DOI] [PubMed] [Google Scholar]


