Abstract
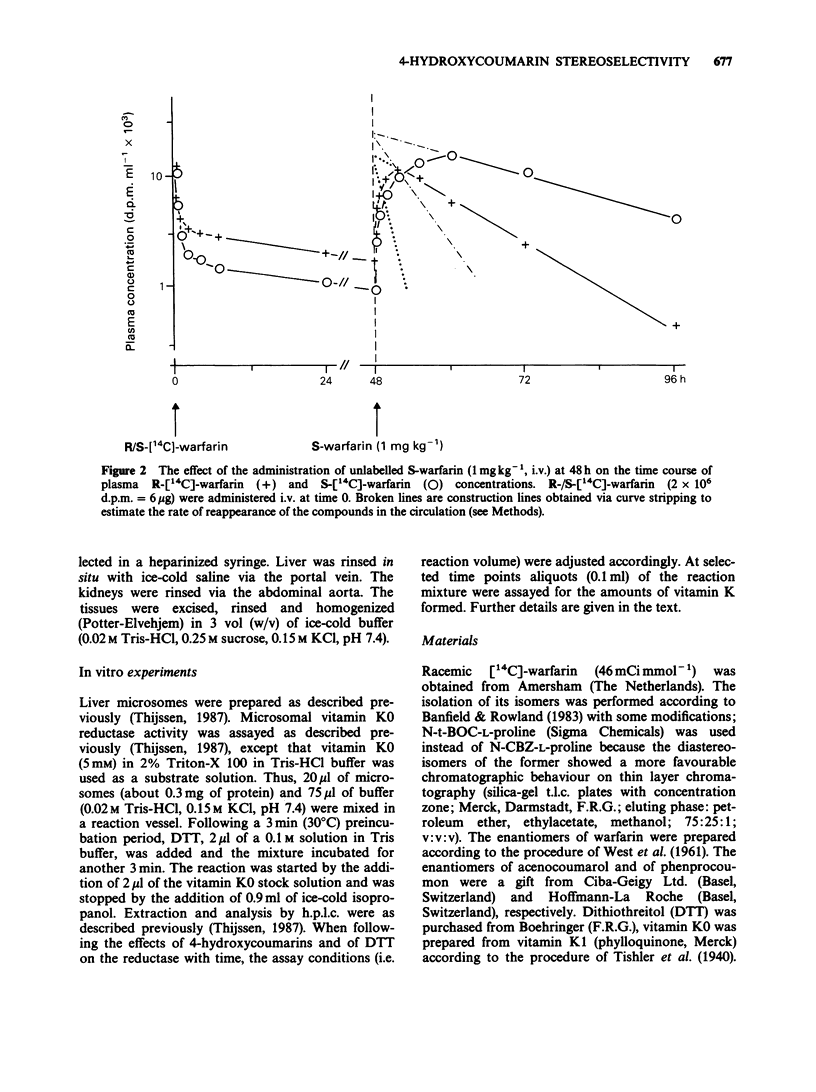
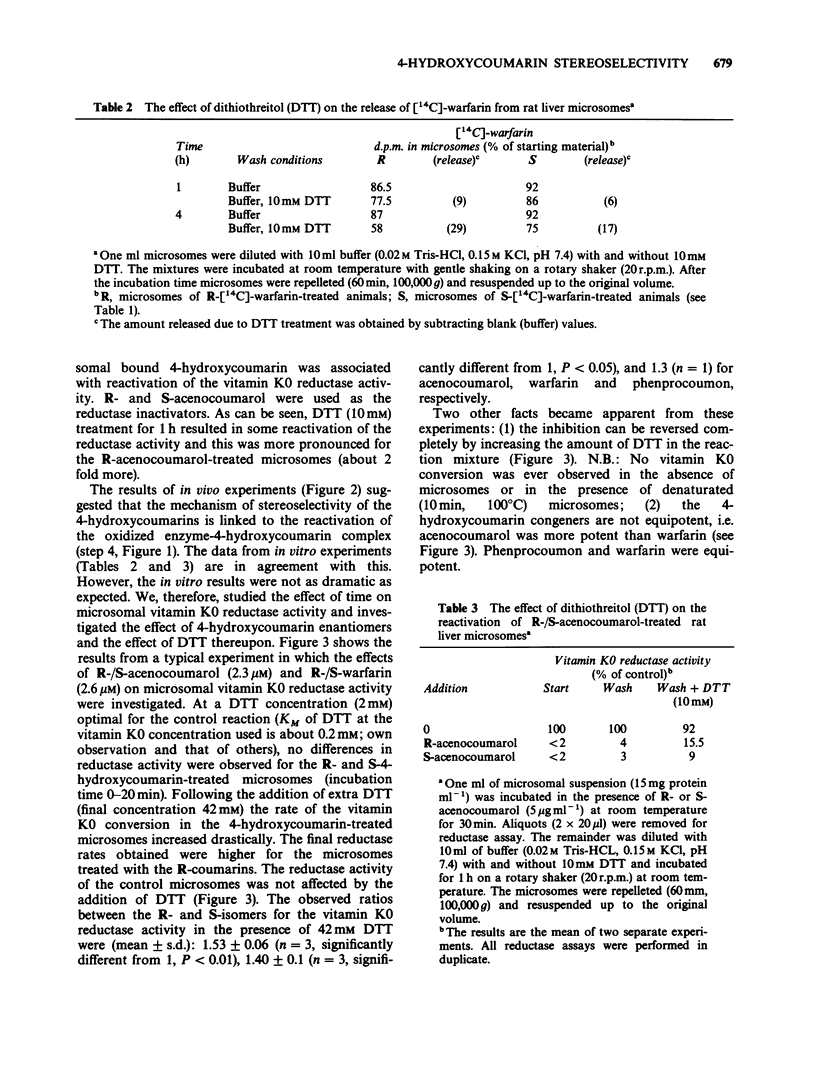
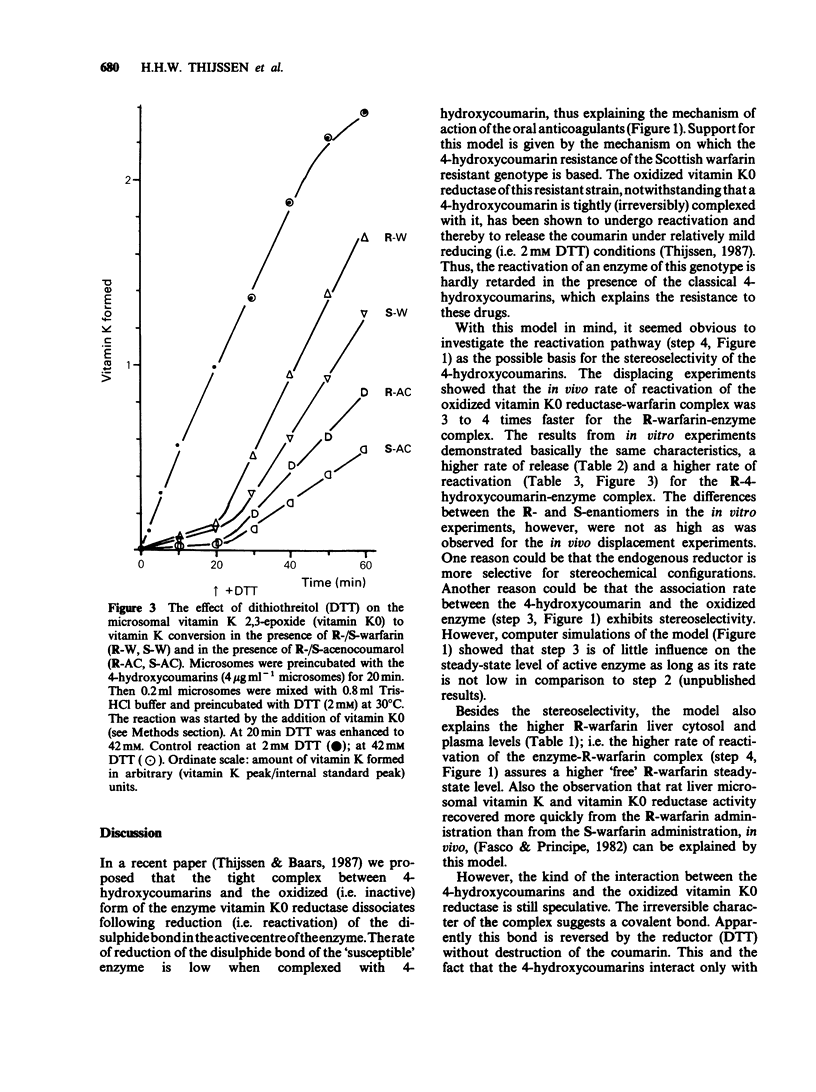
1. The administration of S-warfarin (1 mg kg-1 i.v.) to rats that were pre-loaded 48 h before with tracer doses (6 micrograms) of 14C-labelled R- or S-warfarin caused the plasma levels of these compounds to increase. This is due to the substitution of the microsomal (vitamin K 2,3-epoxide (K0) reductase) bound R- or S-[14C]-warfarin by the unlabelled 4-hydroxycoumarin administered. The rate of reappearance was 3-4 fold higher for R- than for S-warfarin; t1/2 of release: 1.2 +/- 0.04 and 3.7 +/- 0.6 h, respectively. 2. Liver microsomes prepared from rats pretreated with R- or S-[14C]-warfarin, released these compounds only in the presence of dithiothreitol (DTT; 10 mM). The rate of release was higher for R- than for S-warfarin-treated microsomes. 3. Liver microsomes treated in vitro with R- or S-acenocoumarol could be reactivated by DTT (10 mM). Reactivation was higher for the R- than for the S-acenocoumarol-treated microsomes. 4. The microsomal vitamin K0 reductase activity under 'normal' assay conditions ([DTT] = 2 mM) was as sensitive for R- as for S-4-hydroxycoumarins. At elevated DTT concentrations (= 42 mM) the rate of vitamin K0 conversion was about 1.5 fold higher in the presence of the R-isomers than in the presence of the S-isomers. For instance, at 2 mM DDT the reductase activities in the presence of 2.6 microM R- and S-warfarin were about 15% of control. At 42 mM DTT the activities were 90 and 65% of control, respectively. 5. In the in vitro experiments acenocoumarol appeared to be more potent than warfarin and phenprocoumon. 6. The following mechanism is proposed: vitamin K0 reductase becomes oxidized during substrate reduction.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banfield C., Rowland M. Stereospecific high-performance liquid chromatographic analysis of warfarin in plasma. J Pharm Sci. 1983 Aug;72(8):921–924. doi: 10.1002/jps.2600720820. [DOI] [PubMed] [Google Scholar]
- Fasco M. J., Principe L. M. R- and S-Warfarin inhibition of vitamin K and vitamin K 2,3-epoxide reductase activities in the rat. J Biol Chem. 1982 May 10;257(9):4894–4901. [PubMed] [Google Scholar]
- Fasco M. J., Principe L. M., Walsh W. A., Friedman P. A. Warfarin inhibition of vitamin K 2,3-epoxide reductase in rat liver microsomes. Biochemistry. 1983 Nov 22;22(24):5655–5660. doi: 10.1021/bi00293a031. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Fasco M. J. Metabolism of vitamin K and vitamin K 2,3-epoxide via interaction with a common disulfide. Biochemistry. 1984 May 8;23(10):2246–2252. doi: 10.1021/bi00305a024. [DOI] [PubMed] [Google Scholar]
- Meinertz T., Kasper W., Kahl C., Jähnchen E. Anticoagulant activity of the enantiomers of acenocoumarol. Br J Clin Pharmacol. 1978 Feb;5(2):187–188. doi: 10.1111/j.1365-2125.1978.tb01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly R. A. Studies on the optical enantiomorphs of warfarin in man. Clin Pharmacol Ther. 1974 Aug;16(2):348–354. doi: 10.1002/cpt1974162348. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Jähnchen E. Stereoselective drug distribution and anticoagulant potency of the enantiomers of phenprocoumon in rats. J Pharm Pharmacol. 1977 May;29(5):266–271. doi: 10.1111/j.2042-7158.1977.tb11309.x. [DOI] [PubMed] [Google Scholar]
- Suttie J. W. The metabolic role of vitamin K. Fed Proc. 1980 Aug;39(10):2730–2735. [PubMed] [Google Scholar]
- Thijssen H. H., Baars L. G., Drittij-Reijnders M. J. Stereoselective aspects in the pharmacokinetics and pharmacodynamics of acenocoumarol and its amino and acetamido derivatives in the rat. Drug Metab Dispos. 1985 Sep-Oct;13(5):593–597. [PubMed] [Google Scholar]
- Thijssen H. H., Baars L. G. Hepatic uptake and storage of warfarin. The relation with the target enzyme vitamin K 2,3-epoxide reductase. J Pharmacol Exp Ther. 1987 Dec;243(3):1082–1088. [PubMed] [Google Scholar]
- Thijssen H. H., Janssen C. A., Drittij-Reijnders M. J. The effect of S-warfarin administration on vitamin K 2,3-epoxide reductase activity in liver, kidney and testis of the rat. Biochem Pharmacol. 1986 Oct 1;35(19):3277–3282. doi: 10.1016/0006-2952(86)90424-7. [DOI] [PubMed] [Google Scholar]
- Thijssen H. H., Janssen G. M., Baars L. G. Lack of effect of cimetidine on pharmacodynamics and kinetics of single oral doses of R- and S-acenocoumarol. Eur J Clin Pharmacol. 1986;30(5):619–623. doi: 10.1007/BF00542424. [DOI] [PubMed] [Google Scholar]
- Thijssen H. H. Warfarin resistance. Vitamin K epoxide reductase of Scottish resistance genes is not irreversibly blocked by warfarin. Biochem Pharmacol. 1987 Sep 1;36(17):2753–2757. doi: 10.1016/0006-2952(87)90260-7. [DOI] [PubMed] [Google Scholar]
- Wilson A. C., Fasco M. J. Vitamin-K-dependent proteins in microsomes of primary Lewis lung tumors. Int J Cancer. 1986 Dec 15;38(6):877–882. doi: 10.1002/ijc.2910380615. [DOI] [PubMed] [Google Scholar]
- Wingard L. B., Jr, O'Reilly R. A., Levy G. Pharmacokinetics of warfarin enantiomers: a search for intrasubject correlations. Clin Pharmacol Ther. 1978 Feb;23(2):212–217. doi: 10.1002/cpt1978232212. [DOI] [PubMed] [Google Scholar]
- Yacobi A., Levy G. Pharmacokinetics of the warfarin enantiomers in rats. J Pharmacokinet Biopharm. 1974 Jun;2(3):239–255. doi: 10.1007/BF01059764. [DOI] [PubMed] [Google Scholar]


