Abstract
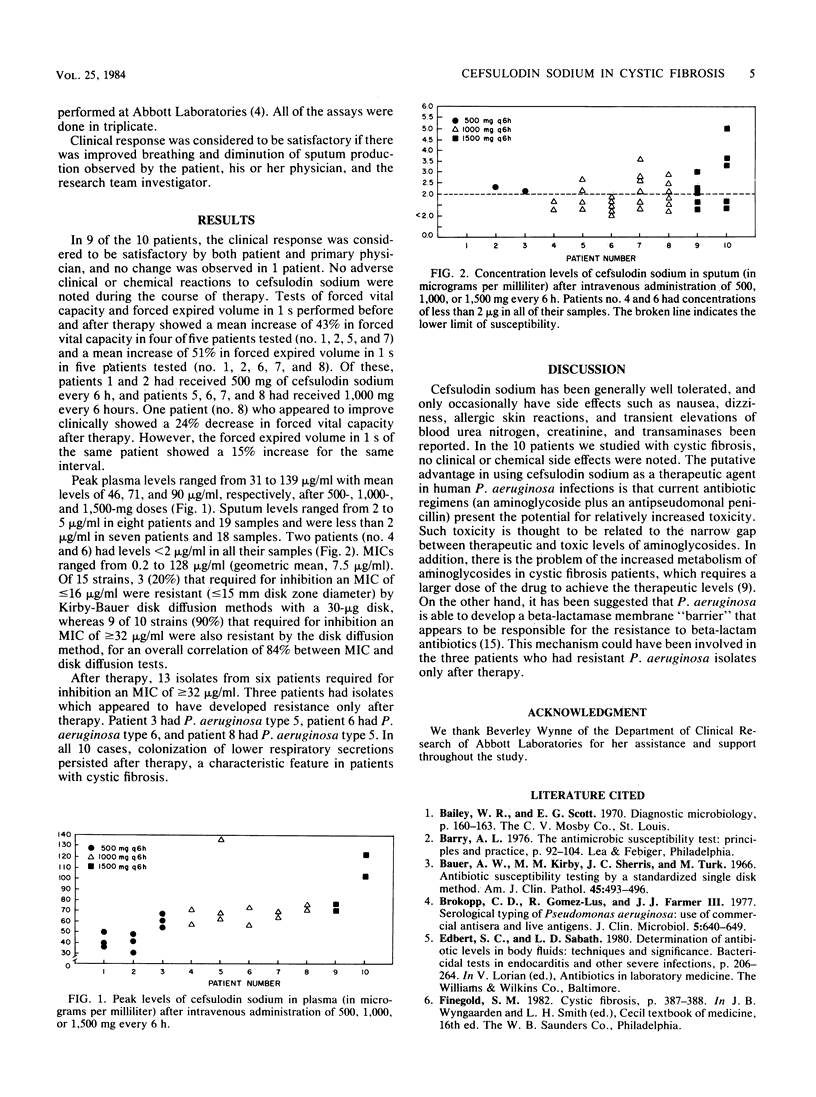
Cefsulodin sodium is a narrow-spectrum cephalosporin with marked in vitro activity against clinical isolates of Pseudomonas aeruginosa. We have studied the antibiotic in a clinical trial in 10 patients admitted to the Pediatric Ward of the University of Virginia Medical Center with cystic fibrosis and recurrent acute lower respiratory tract infections with P. aeruginosa isolated from their sputa. The patients received 500 to 1,500 mg of cefsulodin every 6 hours by intravenous infusion for 10 to 22 days. Mean peak drug levels in plasma after 500, 1,000, and 1,500 mg were 46, 71, and 90 micrograms/ml, respectively, and the mean minimal inhibitory concentration of all organisms was 7.5 micrograms/ml. Detectable levels of cefsulodin in sputa were found in approximately half of the random samples and ranged from 2 to 5 micrograms/ml. The clinical response was satisfactory in nine (90%) of the patients. One patient gained weight and had improved pulmonary function tests but showed no reduction in sputum production and no improvement in arterial blood gas values. In pulmonary function tests, four of five patients tested showed an average 43% increase in forced vital capacity after initiation of therapy and five of five had an average 51% increase in forced expired volume in 1 s. No adverse effects were observed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Brokopp C. D., Gomez-Lus R., Farmer J. J., 3rd Serological typing of Pseudomonas aeruginosa: use of commercial antisera and live antigens. J Clin Microbiol. 1977 Jun;5(6):640–649. doi: 10.1128/jcm.5.6.640-649.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson T. P., Granneman G. R., Kallal J. E., Sennello L. T. Cefsulodin kinetics in renal impairment. Clin Pharmacol Ther. 1982 May;31(5):602–608. doi: 10.1038/clpt.1982.84. [DOI] [PubMed] [Google Scholar]
- Granneman G. R., Sennello L. T., Sonders R. C., Wynne B., Thomas E. W. Cefsulodin kinetics in healthy subjects after intramuscular and intravenous injection. Clin Pharmacol Ther. 1982 Jan;31(1):95–103. doi: 10.1038/clpt.1982.15. [DOI] [PubMed] [Google Scholar]
- Kearns G. L., Hilman B. C., Wilson J. T. Dosing implications of altered gentamicin disposition in patients with cystic fibrosis. J Pediatr. 1982 Feb;100(2):312–318. doi: 10.1016/s0022-3476(82)80663-x. [DOI] [PubMed] [Google Scholar]
- Kettner M., Navarová J., Rýdl Z., Knothe H., Lebek G., Krcméry V. Occurrence of aminoglycoside-modifying enzymes in resistant strains of enterobacteria and Pseudomonas aeruginosa from several countries. J Antimicrob Chemother. 1981 Sep;8(3):175–185. doi: 10.1093/jac/8.3.175. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The in vitro activity, human pharmacology, and clinical effectiveness of new beta-lactam antibiotics. Annu Rev Pharmacol Toxicol. 1982;22:599–642. doi: 10.1146/annurev.pa.22.040182.003123. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The new beta-lactamase-stable cephalosporins. Ann Intern Med. 1982 Sep;97(3):408–419. doi: 10.7326/0003-4819-97-3-408. [DOI] [PubMed] [Google Scholar]
- Norrby R. Current status of Pseudomonas infections and antibiotics. Scand J Infect Dis Suppl. 1981;29:81–86. [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Emergence of resistance during therapy with the newer beta-lactam antibiotics: role of inducible beta-lactamases and implications for the future. Rev Infect Dis. 1983 Jul-Aug;5(4):639–648. doi: 10.1093/clinids/5.4.639. [DOI] [PubMed] [Google Scholar]


