Abstract
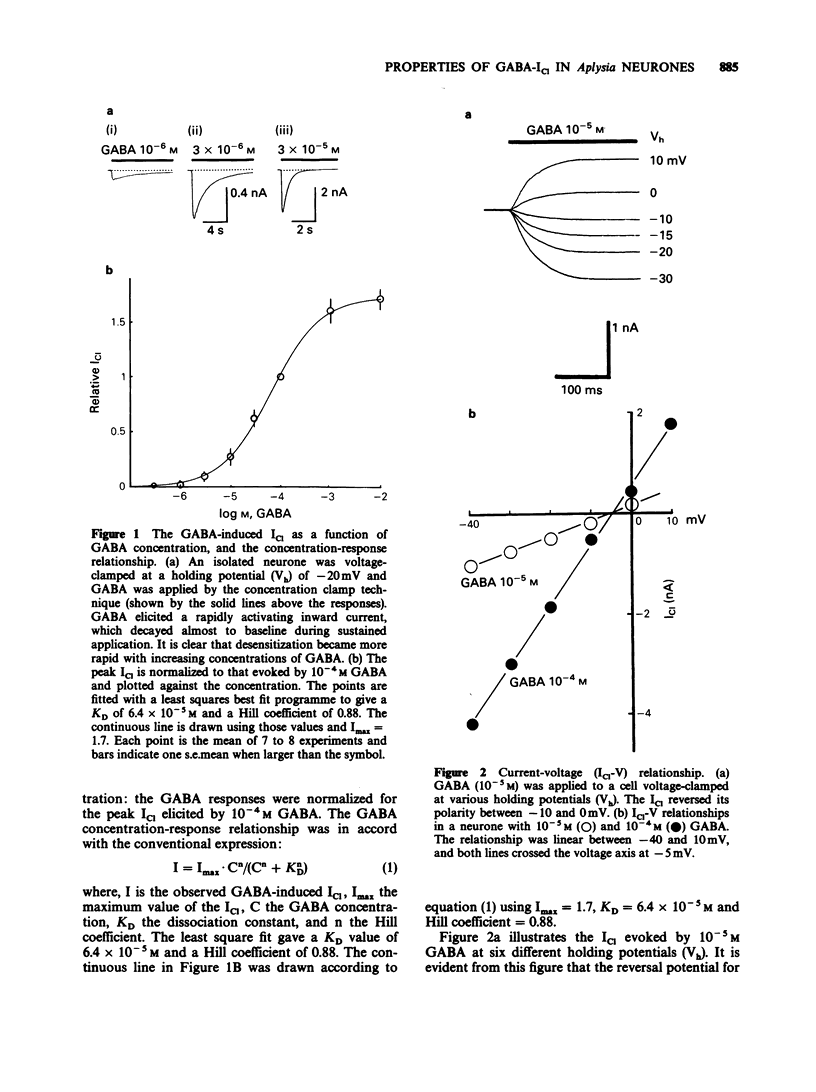
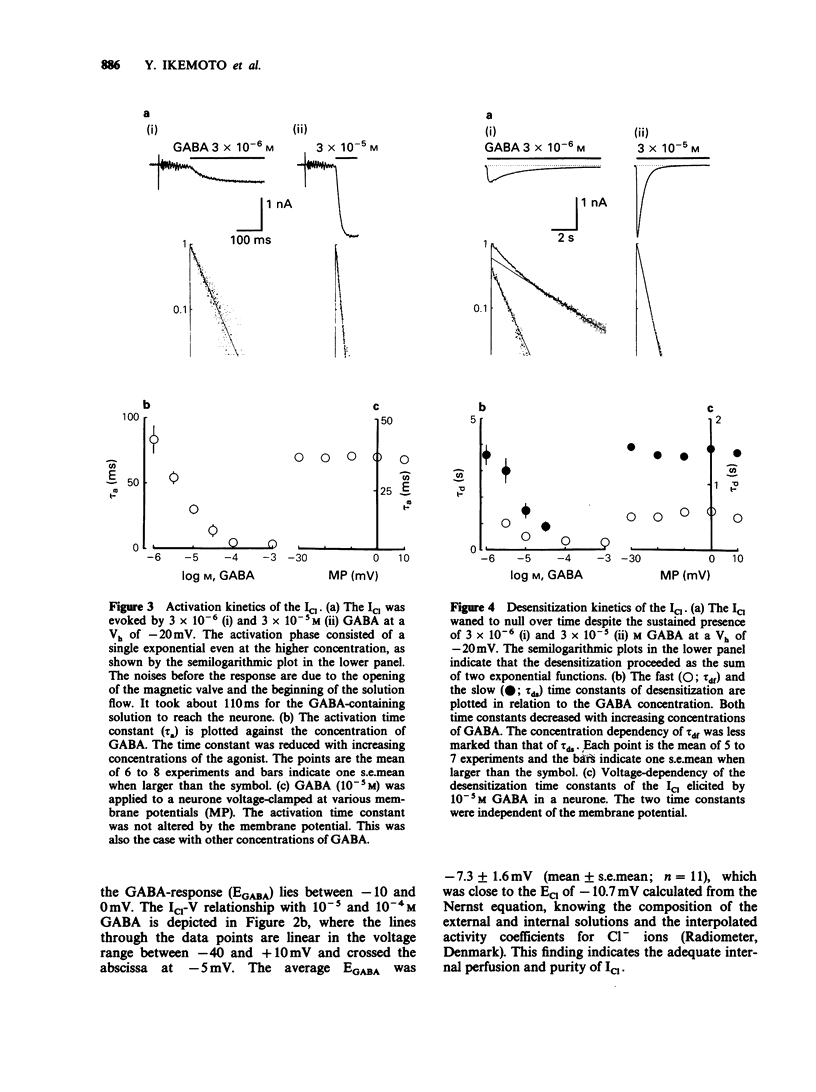
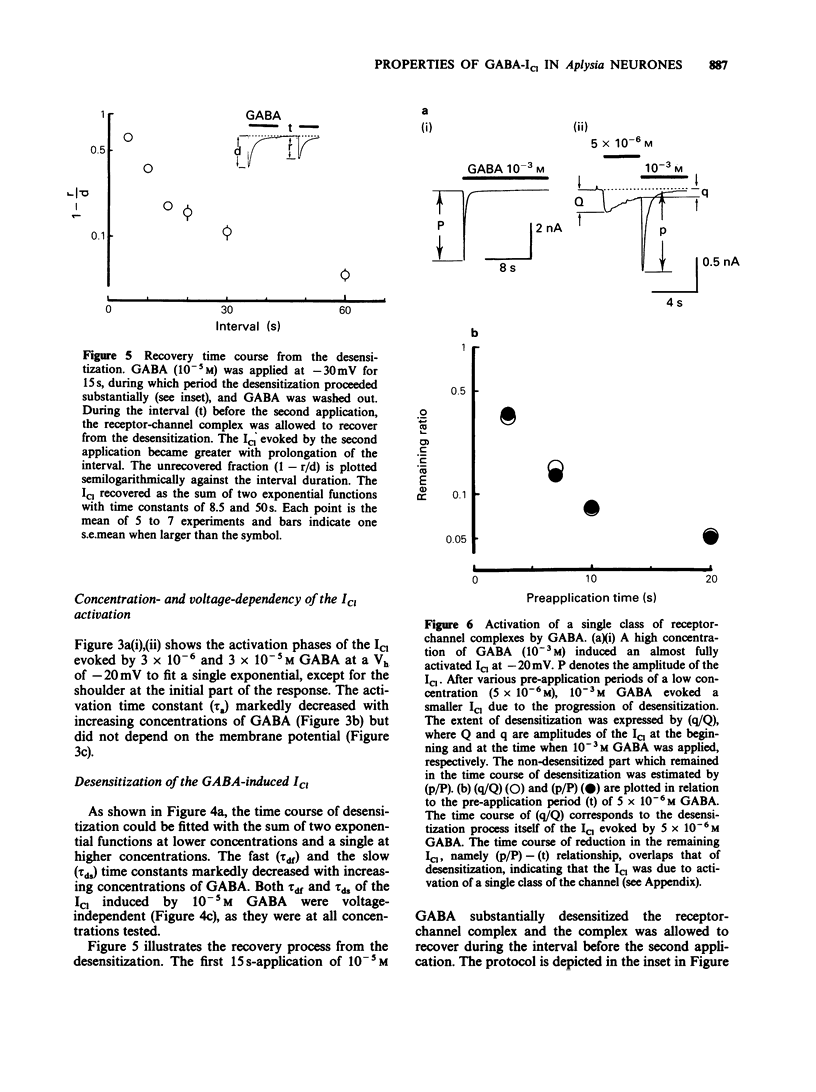
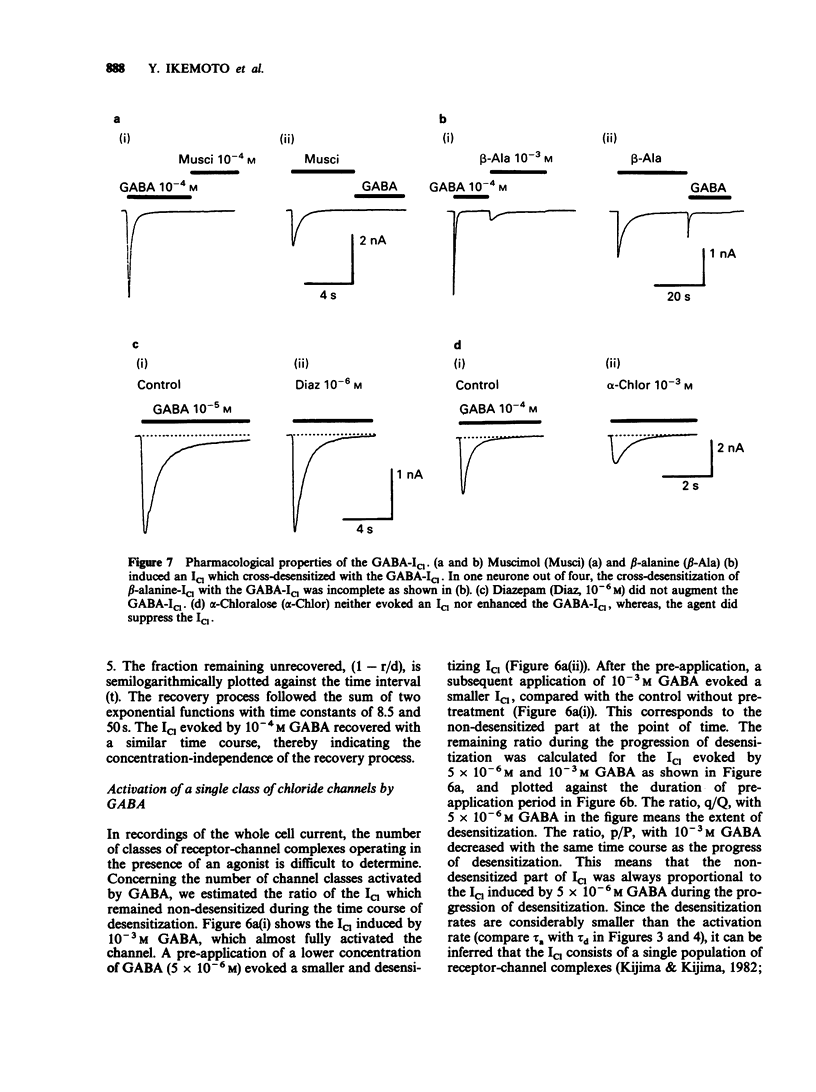
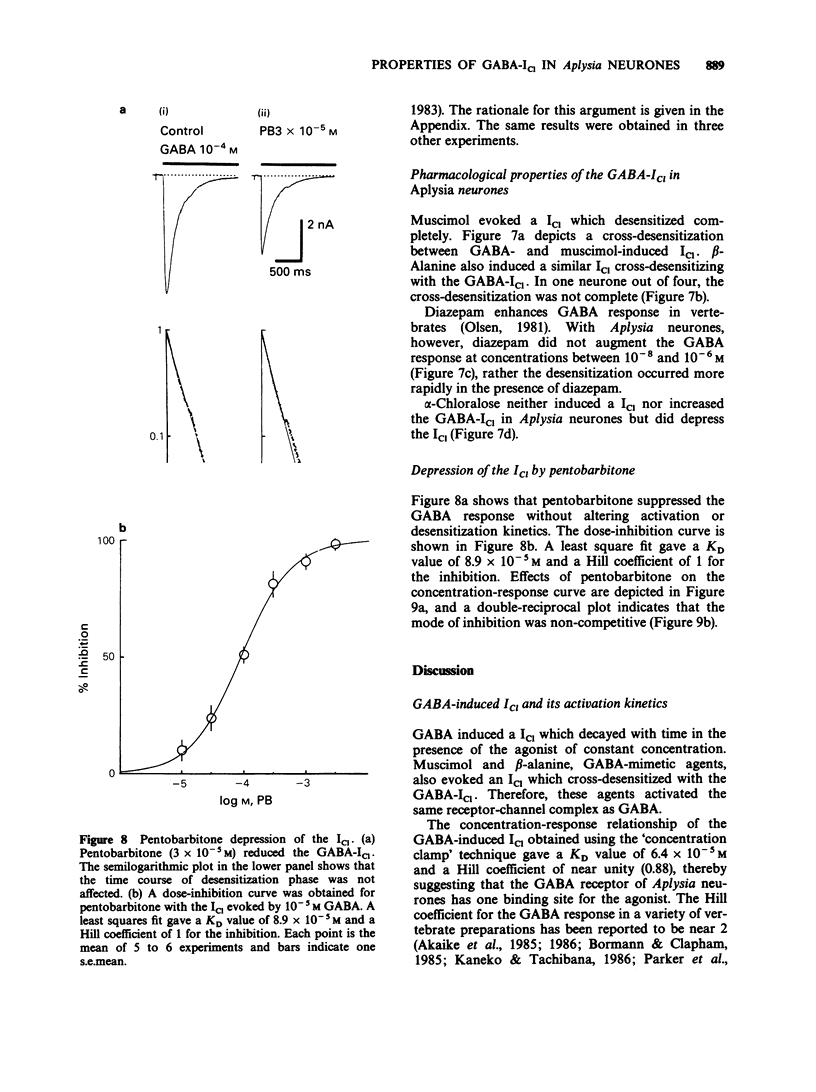
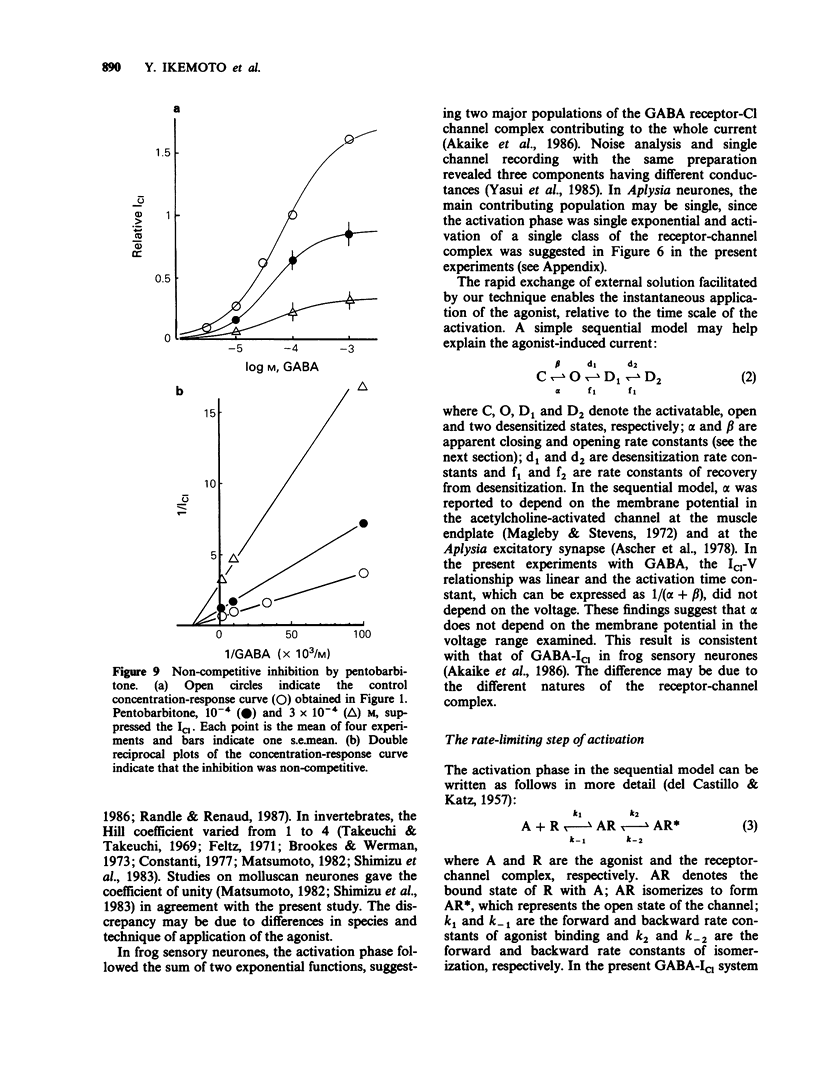
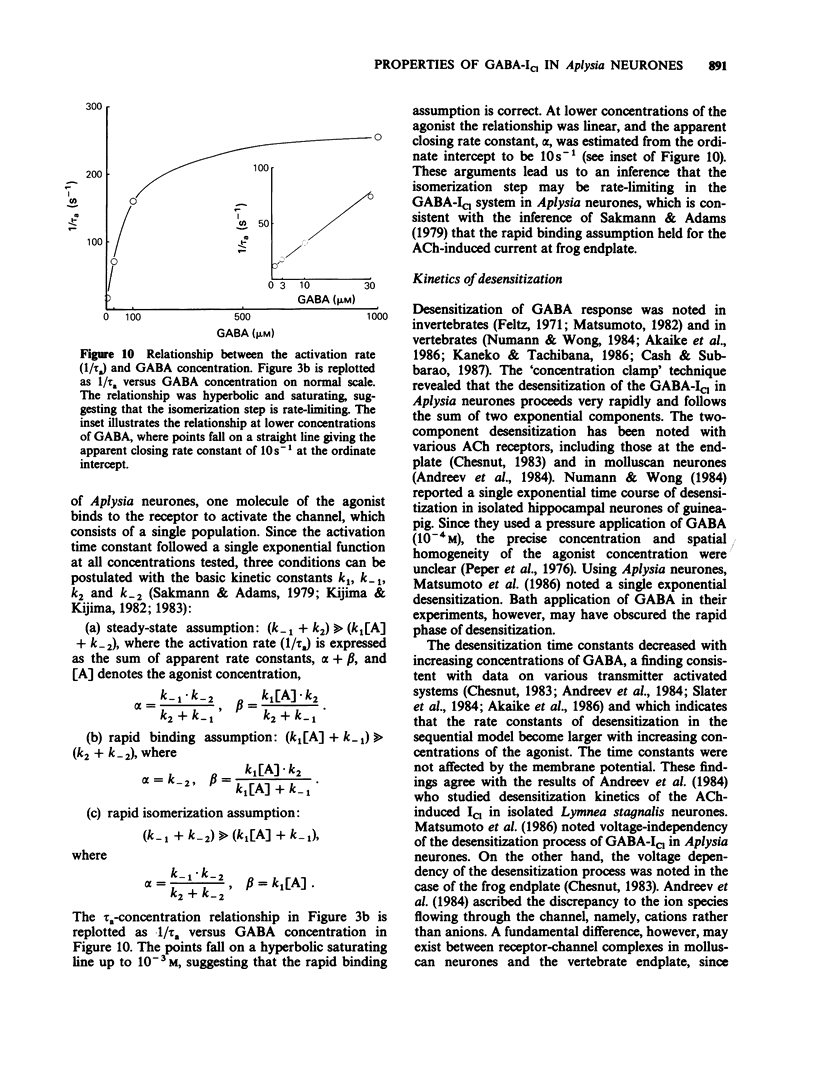
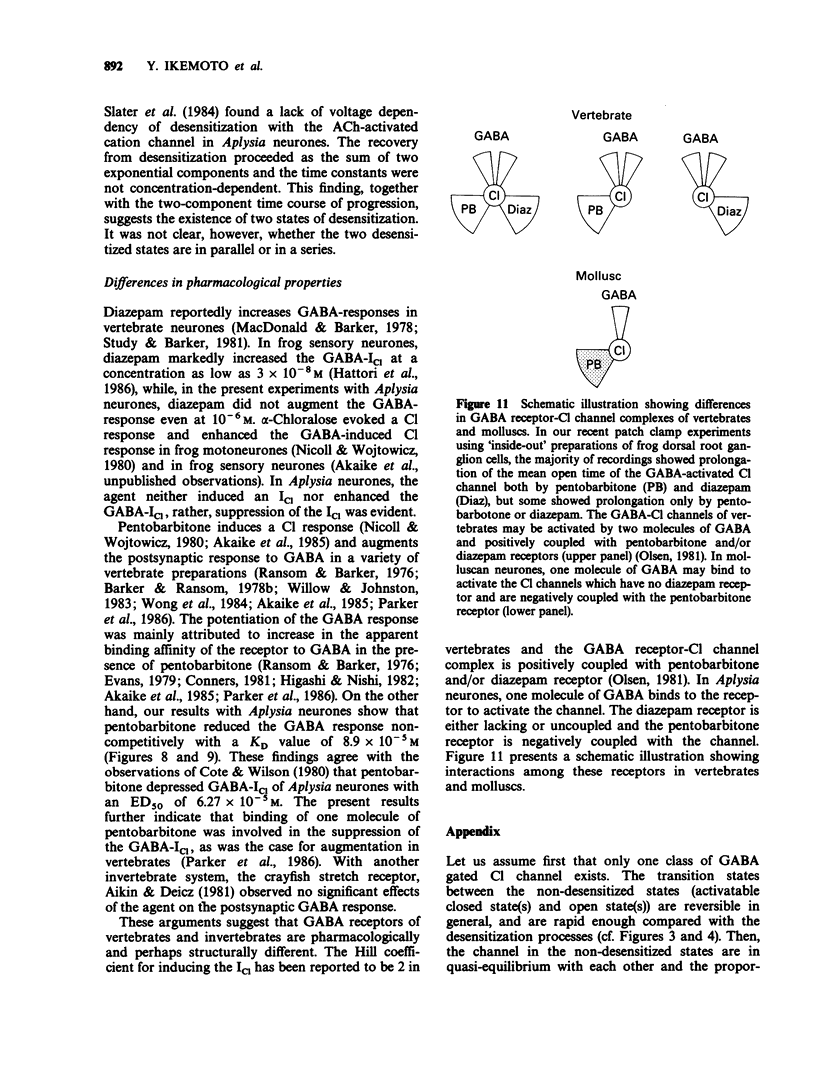
1. gamma-Aminobutyric acid (GABA) was applied by the 'concentration clamp' technique to isolated neurones of Aplysia. GABA induced a chloride current (ICl) due to activation of a single class of chloride-channel. 2. The concentration-response curve for the peak ICl gave an apparent dissociation constant of 6.4 X 10(-5) M and a Hill coefficient of 0.88. The current-voltage relationship was linear in the voltage range examined (-40 to +10 mV). 3. The activation phase of the ICl could be fitted to a single exponential function and desensitization followed the sum of two exponential functions. The time constants of activation and desensitization decreased with increasing concentrations of GABA but were voltage-independent. The recovery process from desensitization also followed the sum of two exponential functions. 4. As for the rate-limiting step of the channel activation, the hyperbolic relationship between the activation rate and GABA concentration showed that the rapid binding assumption holds, suggesting that the isomerization step is rate-limiting. The apparent channel closing rate constant was estimated to be 10 s-1 from the ordinate intercept of the linear part of the above relationship at lower concentrations. 5. Muscimol and beta-alanine induced a ICl, which cross-desensitized with that evoked by GABA. The GABA-ICl was not enhanced by diazepam (10(-6) M) or alpha-chloralose (10(-3) M), in fact depressant effects were evident. 6. Pentobarbitone decreased the GABA-ICl non-competitively without altering activation or desensitization kinetics. The concentration-inhibition curve gave a KD value of 8.9 x 10(-5) M and a Hill coefficient of 1.0. 7. These results suggest that GABA activates a single class of Cl channel in Aplysia neurones, which have one binding site for the agonist. The GABA receptor-Cl channel complex in Aplysia is pharmacologically and perhaps structurally different from that in vertebrates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Deisz R. A. Pentobarbitone interference with inhibitory synaptic transmission in crayfish stretch receptor neurones. J Physiol. 1981 Jun;315:175–187. doi: 10.1113/jphysiol.1981.sp013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Hattori K., Inomata N., Oomura Y. gamma-Aminobutyric-acid- and pentobarbitone-gated chloride currents in internally perfused frog sensory neurones. J Physiol. 1985 Mar;360:367–386. doi: 10.1113/jphysiol.1985.sp015622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Inoue M., Krishtal O. A. 'Concentration-clamp' study of gamma-aminobutyric-acid-induced chloride current kinetics in frog sensory neurones. J Physiol. 1986 Oct;379:171–185. doi: 10.1113/jphysiol.1986.sp016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev A. A., Veprintsev B. N., Vulfius C. A. Two-component desensitization of nicotinic receptors induced by acetylcholine agonists in Lymnaea stagnalis neurones. J Physiol. 1984 Aug;353:375–391. doi: 10.1113/jphysiol.1984.sp015341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Amino acid pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:331–354. doi: 10.1113/jphysiol.1978.sp012387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Pentobarbitone pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:355–372. doi: 10.1113/jphysiol.1978.sp012388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes N. Werman R:The cooperativity of -aminobutyric action on the membrane of locust muscle fibers. Mol Pharmacol. 1973 Jul;9(4):571–579. [PubMed] [Google Scholar]
- Cash D. J., Subbarao K. gamma-Aminobutyric acid (GABA) mediated transmembrane chloride flux with membrane vesicles from rat brain measured by quench flow technique: kinetic homogeneity of ion flux and receptor desensitization. Life Sci. 1987 Jul 27;41(4):437–445. doi: 10.1016/0024-3205(87)90219-0. [DOI] [PubMed] [Google Scholar]
- Chesnut T. J. Two-component desensitization at the neuromuscular junction of the frog. J Physiol. 1983 Mar;336:229–241. doi: 10.1113/jphysiol.1983.sp014578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Connors B. W. A comparison of the effects of pentobarbital and diphenylhydantoin on the GABA sensitivity and excitability of adult sensory ganglion cells. Brain Res. 1981 Mar 2;207(2):357–369. doi: 10.1016/0006-8993(81)90370-x. [DOI] [PubMed] [Google Scholar]
- Cote I. L., Wilson W. A. Effects of barbiturates on inhibitory and excitatory responses to applied neurotransmitters in Aplysia. J Pharmacol Exp Ther. 1980 Jul;214(1):161–165. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Evans R. H. Potentiation of the effects of GABA by pentobarbitone. Brain Res. 1979 Jul 27;171(1):113–120. doi: 10.1016/0006-8993(79)90736-4. [DOI] [PubMed] [Google Scholar]
- Feltz A. Competitive interaction of beta-guanidino propionic acid and gamma-aminobutyric acid on the muscle fibre of the crayfish. J Physiol. 1971 Jul;216(2):391–401. doi: 10.1113/jphysiol.1971.sp009531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K., Akaike N., Oomura Y., Kuraoka S. Internal perfusion studies demonstrating GABA-induced chloride responses in frog primary afferent neurons. Am J Physiol. 1984 Mar;246(3 Pt 1):C259–C265. doi: 10.1152/ajpcell.1984.246.3.C259. [DOI] [PubMed] [Google Scholar]
- Hattori K., Oomura Y., Akaike N. Diazepam action on gamma-aminobutyric acid-activated chloride currents in internally perfused frog sensory neurons. Cell Mol Neurobiol. 1986 Sep;6(3):307–323. doi: 10.1007/BF00711116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H., Nishi S. Effect of barbiturates on the GABA receptor of cat primary afferent neurones. J Physiol. 1982 Nov;332:299–314. doi: 10.1113/jphysiol.1982.sp014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto Y., Akaike N., Ono K. 4-aminopyridine activates a cholinergic chloride conductance in isolated Helix neurons. Neurosci Lett. 1987 Apr 23;76(1):42–46. doi: 10.1016/0304-3940(87)90189-3. [DOI] [PubMed] [Google Scholar]
- Ishizuka S., Hattori K., Akaike N. Separation of ionic currents in the somatic membrane of frog sensory neurons. J Membr Biol. 1984;78(1):19–28. doi: 10.1007/BF01872528. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Effects of gamma-aminobutyric acid on isolated cone photoreceptors of the turtle retina. J Physiol. 1986 Apr;373:443–461. doi: 10.1113/jphysiol.1986.sp016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima H., Kijima S. 'Steady/equilibrium approximation' in relaxation and fluctuation. I. Procedure to simplify first-order reaction. Biophys Chem. 1982 Nov;16(3):181–192. doi: 10.1016/0301-4622(82)87001-4. [DOI] [PubMed] [Google Scholar]
- Kijima H., Kijima S. 'Steady/equilibrium approximation' in relaxation and fluctuation. II. Mathematical theory of approximations in first-order reaction. Biophys Chem. 1983 Jun;17(4):261–283. doi: 10.1016/0301-4622(83)80012-x. [DOI] [PubMed] [Google Scholar]
- Macdonald R., Barker J. L. Benzodiazepines specifically modulate GABA-mediated postsynaptic inhibition in cultured mammalian neurones. Nature. 1978 Feb 9;271(5645):563–564. doi: 10.1038/271563a0. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Sasaki K., Shozushima M., Sato M. Desensitization of Cl(-)-dependent GABA response observed in ganglion cells of Aplysia. Jpn J Physiol. 1986;36(2):349–358. doi: 10.2170/jjphysiol.36.349. [DOI] [PubMed] [Google Scholar]
- Matsumoto M. The voltage-dependent nature of the GABA-induced conductance change recorded from the ganglion cell of Aplysia. Jpn J Physiol. 1982;32(1):55–67. doi: 10.2170/jjphysiol.32.55. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Wojtowicz J. M. The effects of pentobarbital and related compounds on frog motoneurons. Brain Res. 1980 Jun 2;191(1):225–237. doi: 10.1016/0006-8993(80)90325-x. [DOI] [PubMed] [Google Scholar]
- Numann R. E., Wong R. K. Voltage-clamp study on GABA response desensitization in single pyramidal cells dissociated from the hippocampus of adult guinea pigs. Neurosci Lett. 1984 Jun 29;47(3):289–294. doi: 10.1016/0304-3940(84)90528-7. [DOI] [PubMed] [Google Scholar]
- Olsen R. W. GABA-benzodiazepine-barbiturate receptor interactions. J Neurochem. 1981 Jul;37(1):1–13. doi: 10.1111/j.1471-4159.1981.tb05284.x. [DOI] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. Actions of pentobarbital on rat brain receptors expressed in Xenopus oocytes. J Neurosci. 1986 Aug;6(8):2290–2297. doi: 10.1523/JNEUROSCI.06-08-02290.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper K., Dreyer F., Müller K. D. Analysis of cooperativity of drug-receptor interaction by quantitative iontophoresis at frog motor end plates. Cold Spring Harb Symp Quant Biol. 1976;40:187–192. doi: 10.1101/sqb.1976.040.01.020. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Renaud L. P. Actions of gamma-aminobutyric acid on rat supraoptic nucleus neurosecretory neurones in vitro. J Physiol. 1987 Jun;387:629–647. doi: 10.1113/jphysiol.1987.sp016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom B. R., Barker J. L. Pentobarbital selectively enhances GABA-mediated post-synaptic inhibition in tissue cultured mouse spinal neurons. Brain Res. 1976 Sep 24;114(3):530–535. doi: 10.1016/0006-8993(76)90977-x. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Akaike N., Oomura Y., Maruhashi J., Klee M. R. GABA and lioresal actions on the identified Onchidium neuron. Jpn J Physiol. 1983;33(3):459–467. doi: 10.2170/jjphysiol.33.459. [DOI] [PubMed] [Google Scholar]
- Slater N. T., Hall A. F., Carpenter D. O. Kinetic properties of cholinergic desensitization in Aplysia neurons. Proc R Soc Lond B Biol Sci. 1984 Nov 22;223(1230):63–78. doi: 10.1098/rspb.1984.0083. [DOI] [PubMed] [Google Scholar]
- Study R. E., Barker J. L. Diazepam and (--)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. A study of the action of picrotoxin on the inhibitory neuromuscular junction of the crayfish. J Physiol. 1969 Nov;205(2):377–391. doi: 10.1113/jphysiol.1969.sp008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. A study of the inhibitory action of gamma-amino-butyric acid on neuromuscular transmission in the crayfish. J Physiol. 1966 Mar;183(2):418–432. doi: 10.1113/jphysiol.1966.sp007874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willow M., Johnston G. A. Pharmacology of barbiturates: electrophysiological and neurochemical studies. Int Rev Neurobiol. 1983;24:15–49. doi: 10.1016/s0074-7742(08)60219-6. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Leeb-Lundberg L. M., Teichberg V. I., Olsen R. W. gamma-Aminobutyric acid activation of 36Cl- flux in rat hippocampal slices and its potentiation by barbiturates. Brain Res. 1984 Jun 15;303(2):267–275. doi: 10.1016/0006-8993(84)91213-7. [DOI] [PubMed] [Google Scholar]
- Yarowsky P. J., Carpenter D. O. Receptors for gamma-aminobutyric acid (GABA) on Aplysia neurons. Brain Res. 1978 Apr 7;144(1):75–94. doi: 10.1016/0006-8993(78)90436-5. [DOI] [PubMed] [Google Scholar]
- Yasui S., Ishizuka S., Akaike N. GABA activates different types of chloride-conducting receptor-ionophore complexes in a dose-dependent manner. Brain Res. 1985 Sep 30;344(1):176–180. doi: 10.1016/0006-8993(85)91206-5. [DOI] [PubMed] [Google Scholar]


