Abstract
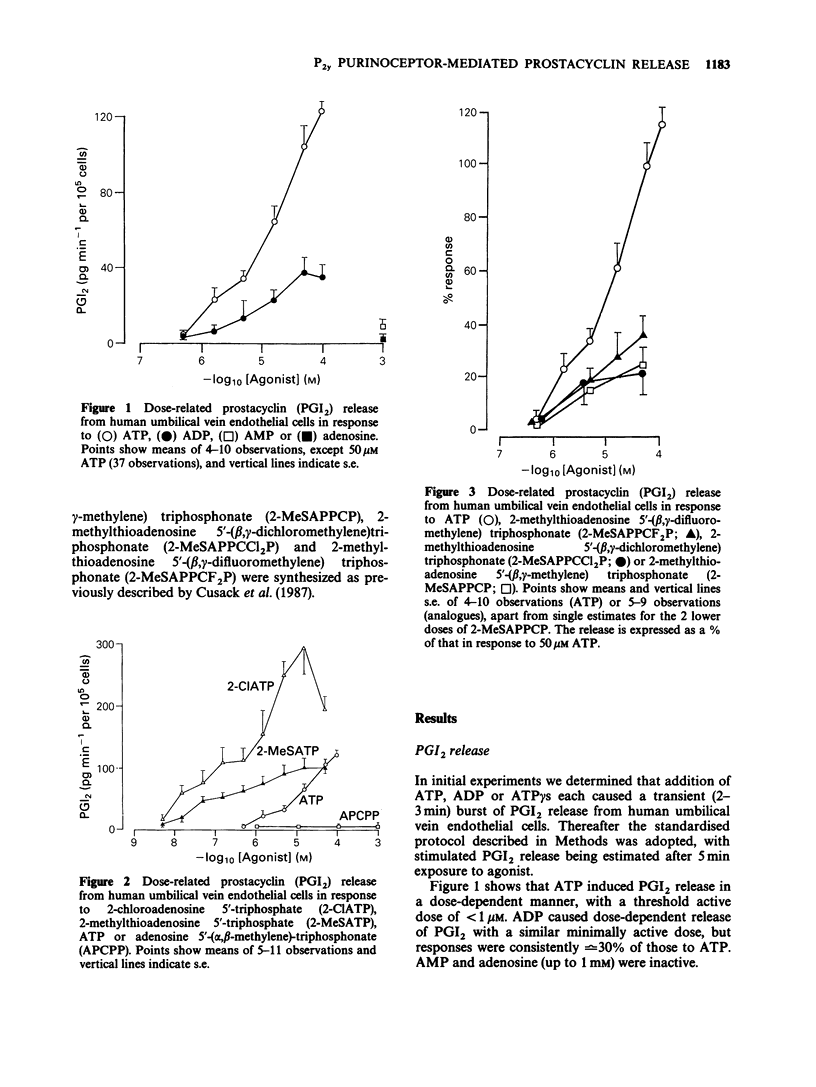
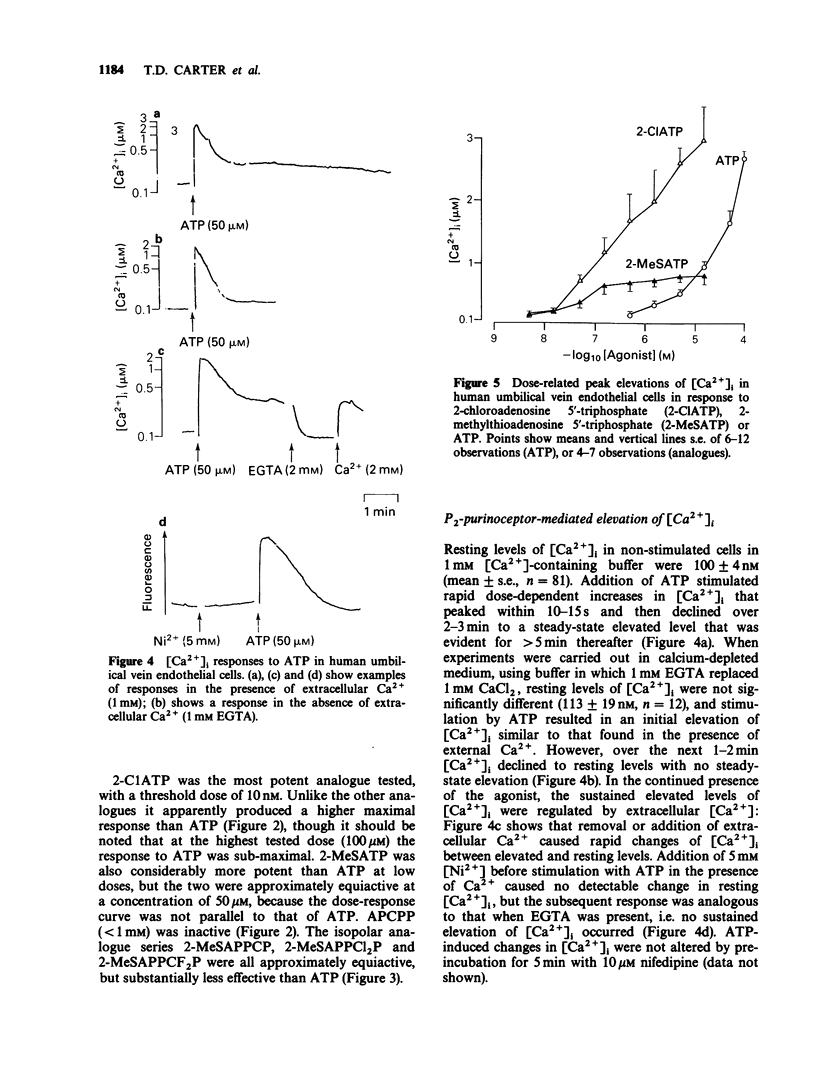
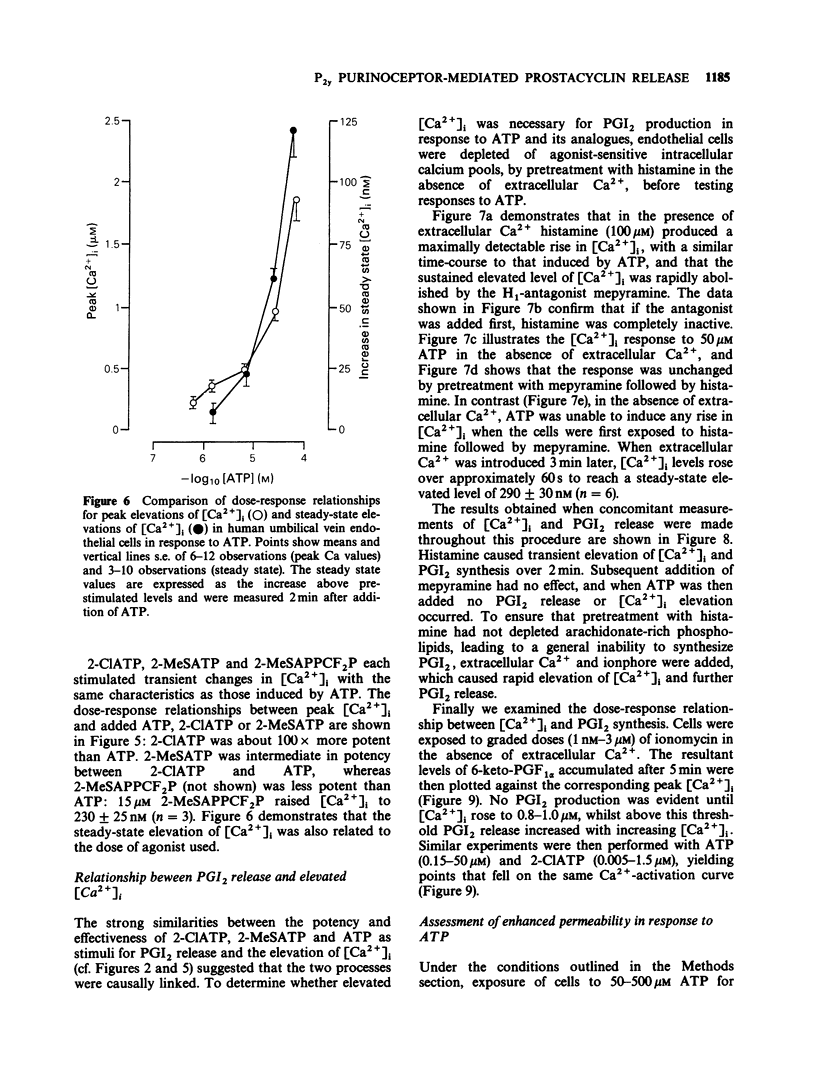
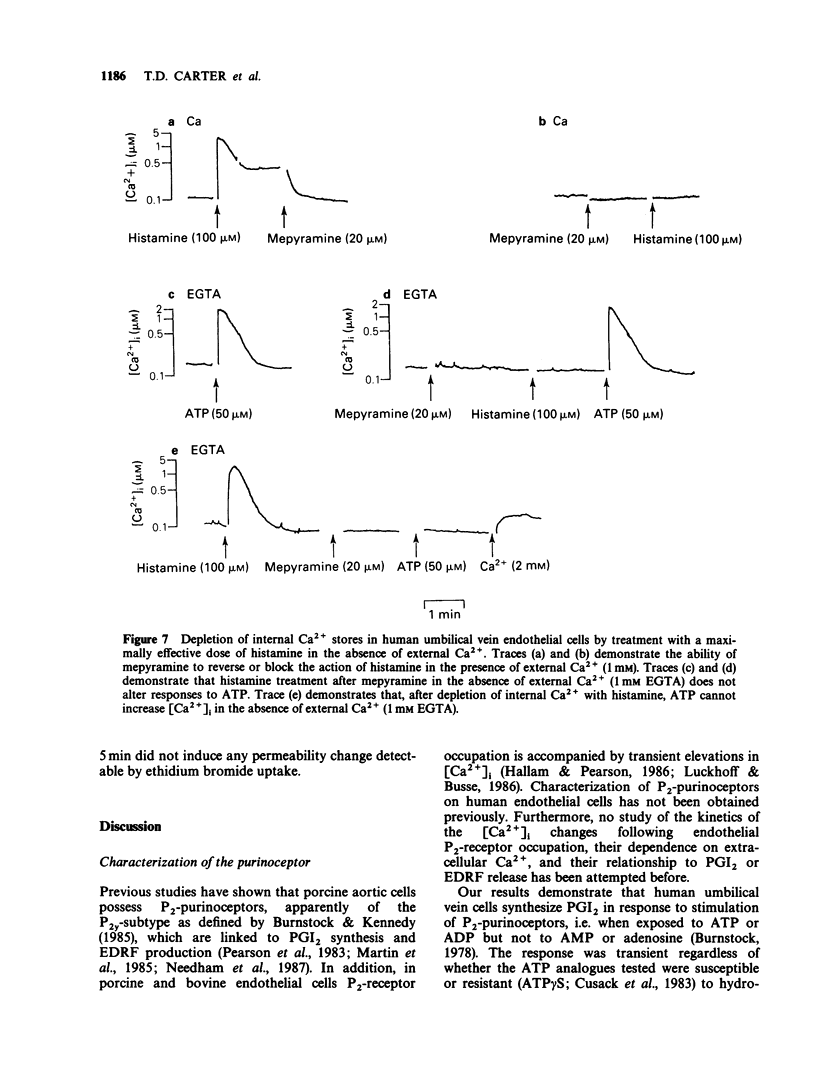
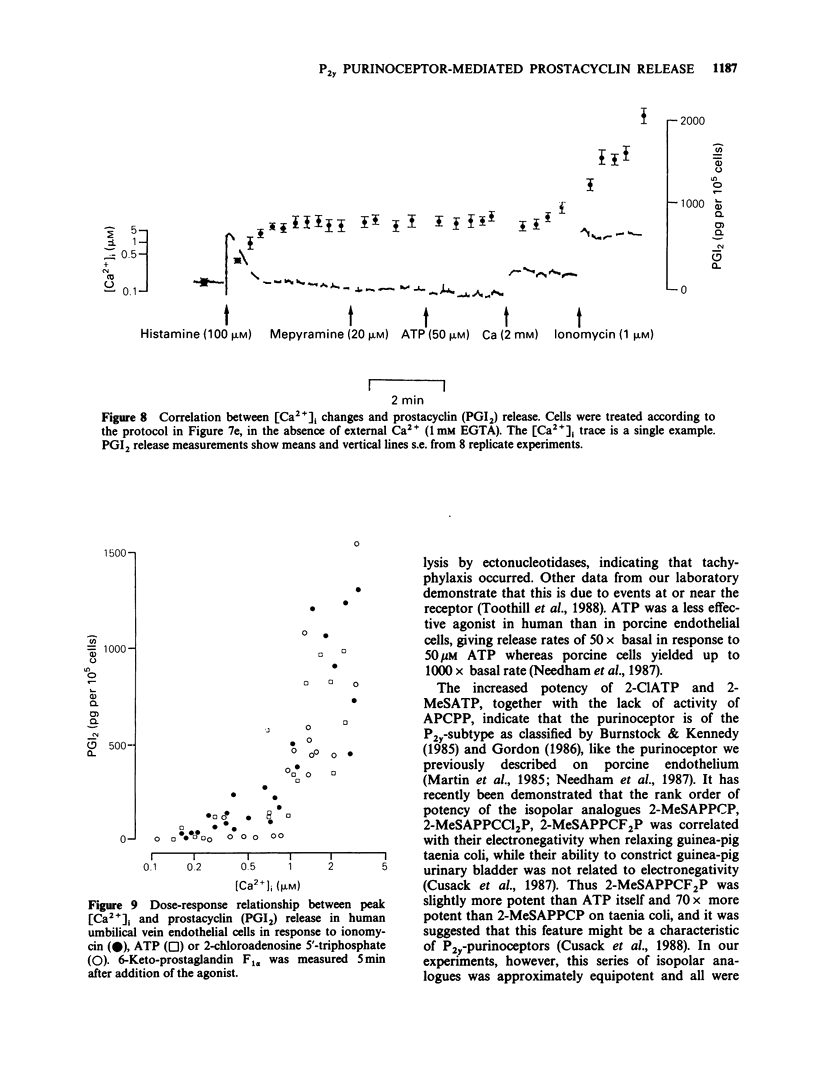
1. ATP and ATP analogues induced prostacyclin (PGI2) secretion from human cultured umbilical vein endothelial cells. 2. The threshold active concentration for ATP was less than or equal to 1 microM. The rank order of potency of analogues was 2-chloroadenosine 5'-triphosphate (2-ClATP) greater than 2-methylthioadenosine 5'-triphosphate (2-MeSATP) greater than ATP greater than ADP, while adenosine 5'-(alpha,beta-methylene)triphosphonate, AMP and adenosine were inactive, indicating the presence of P2y-purinoceptors. 3. In contrast to their actions on P2y-receptors in guinea-pig taenia coli, isopolar analogues of 2-methylthioadenosine 5'-(beta, gamma-methylene)triphosphonate were less effective than ATP. 4. ATP and ATP analogues increased intracellular free calcium ions, [Ca2+]i, giving a rapid transient peak due predominantly to release from intracellular stores, followed by a maintained steady-state elevated level due to influx. 5. The dose-response curves for peak [Ca2+]i induced by ATP, 2-ClATP and 2-MeSATP were very similar to those for PGI2 production. 6. Elevations of [Ca2+]i, above a threshold value of 0.8-1 microM, were necessary for PGI2 production in response to P2y-receptor activation. 7. The dose relationships between PGI2 release and peak [Ca2+]i were equivalent whether [Ca2+]i was raised by ionomycin or via P2y-receptor activation by ATP or 2-ClATP, indicating that elevations of [Ca2+]i provide the major, if not the exclusive intracellular pathway for P2y-purinoceptor-mediated PGI2 synthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Gordon J. L., Moncada S., Pearson J. D., Salmon J. A., Trevethick M. A. Effects of isolation and culture on prostaglandin synthesis by porcine aortic endothelial and smooth muscle cells. J Cell Physiol. 1982 Jan;110(1):9–16. doi: 10.1002/jcp.1041100103. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. The ATP4- receptor of rat mast cells. Biochem J. 1980 Jun 15;188(3):789–798. doi: 10.1042/bj1880789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack N. J., Hourani S. M., Loizou G. D., Welford L. A. Pharmacological effects of isopolar phosphonate analogues of ATP on P2-purinoceptors in guinea-pig taenia coli and urinary bladder. Br J Pharmacol. 1987 Apr;90(4):791–795. doi: 10.1111/j.1476-5381.1987.tb11233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack N. J., Pearson J. D., Gordon J. L. Stereoselectivity of ectonucleotidases on vascular endothelial cells. Biochem J. 1983 Sep 15;214(3):975–981. doi: 10.1042/bj2140975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Role of the intima in cholinergic and purinergic relaxation of isolated canine femoral arteries. J Physiol. 1981 Jul;316:347–355. doi: 10.1113/jphysiol.1981.sp013792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L., Martin W. Endothelium-dependent relaxation of the pig aorta: relationship to stimulation of 86Rb efflux from isolated endothelial cells. Br J Pharmacol. 1983 Jun;79(2):531–541. doi: 10.1111/j.1476-5381.1983.tb11028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough G. R., Maguire M. H., Satchell D. G. Three new adenosine triphosphate analogs. Synthesis and effects on isolated gut. J Med Chem. 1973 Oct;16(10):1188–1190. doi: 10.1021/jm00268a028. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986 Oct 20;207(1):95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D., Needham L. A. Thrombin-stimulated elevation of human endothelial-cell cytoplasmic free calcium concentration causes prostacyclin production. Biochem J. 1988 Apr 1;251(1):243–249. doi: 10.1042/bj2510243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Agonists stimulate divalent cation channels in the plasma membrane of human platelets. FEBS Lett. 1985 Jul 8;186(2):175–179. doi: 10.1016/0014-5793(85)80703-1. [DOI] [PubMed] [Google Scholar]
- Hamilton K. K., Sims P. J. Changes in cytosolic Ca2+ associated with von Willebrand factor release in human endothelial cells exposed to histamine. Study of microcarrier cell monolayers using the fluorescent probe indo-1. J Clin Invest. 1987 Feb;79(2):600–608. doi: 10.1172/JCI112853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Increased free calcium in endothelial cells under stimulation with adenine nucleotides. J Cell Physiol. 1986 Mar;126(3):414–420. doi: 10.1002/jcp.1041260312. [DOI] [PubMed] [Google Scholar]
- Martin W., Cusack N. J., Carleton J. S., Gordon J. L. Specificity of P2-purinoceptor that mediates endothelium-dependent relaxation of the pig aorta. Eur J Pharmacol. 1985 Feb 5;108(3):295–299. doi: 10.1016/0014-2999(85)90452-2. [DOI] [PubMed] [Google Scholar]
- McIntyre T. M., Zimmerman G. A., Satoh K., Prescott S. M. Cultured endothelial cells synthesize both platelet-activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate. J Clin Invest. 1985 Jul;76(1):271–280. doi: 10.1172/JCI111957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Needham L., Cusack N. J., Pearson J. D., Gordon J. L. Characteristics of the P2 purinoceptor that mediates prostacyclin production by pig aortic endothelial cells. Eur J Pharmacol. 1987 Feb 10;134(2):199–209. doi: 10.1016/0014-2999(87)90166-x. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Slakey L. L., Gordon J. L. Stimulation of prostaglandin production through purinoceptors on cultured porcine endothelial cells. Biochem J. 1983 Jul 15;214(1):273–276. doi: 10.1042/bj2140273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotton S., Erneux C., Boeynaems J. M. Dual role of GTP-binding proteins in the control of endothelial prostacyclin. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1113–1120. doi: 10.1016/s0006-291x(87)80185-7. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986 Dec;103(6 Pt 1):2379–2387. doi: 10.1083/jcb.103.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman M., Raz A. Purinergic vs peptidergic stimulation of lipolysis and prostaglandin generation in the perfused rabbit kidney. Biochem Pharmacol. 1982 Aug 1;31(15):2453–2458. doi: 10.1016/0006-2952(82)90053-3. [DOI] [PubMed] [Google Scholar]
- Singer H. A., Peach M. J. Calcium- and endothelial-mediated vascular smooth muscle relaxation in rabbit aorta. Hypertension. 1982 May-Jun;4(3 Pt 2):19–25. [PubMed] [Google Scholar]
- Tatham P. E., Cusack N. J., Gomperts B. D. Characterisation of the ATP4- receptor that mediates permeabilisation of rat mast cells. Eur J Pharmacol. 1988 Feb 16;147(1):13–21. doi: 10.1016/0014-2999(88)90628-0. [DOI] [PubMed] [Google Scholar]
- Van Coevorden A., Boeynaems J. M. Physiological concentrations of ADP stimulate the release of prostacyclin from bovine aortic endothelial cells. Prostaglandins. 1984 Apr;27(4):615–626. doi: 10.1016/0090-6980(84)90097-2. [DOI] [PubMed] [Google Scholar]


