Abstract
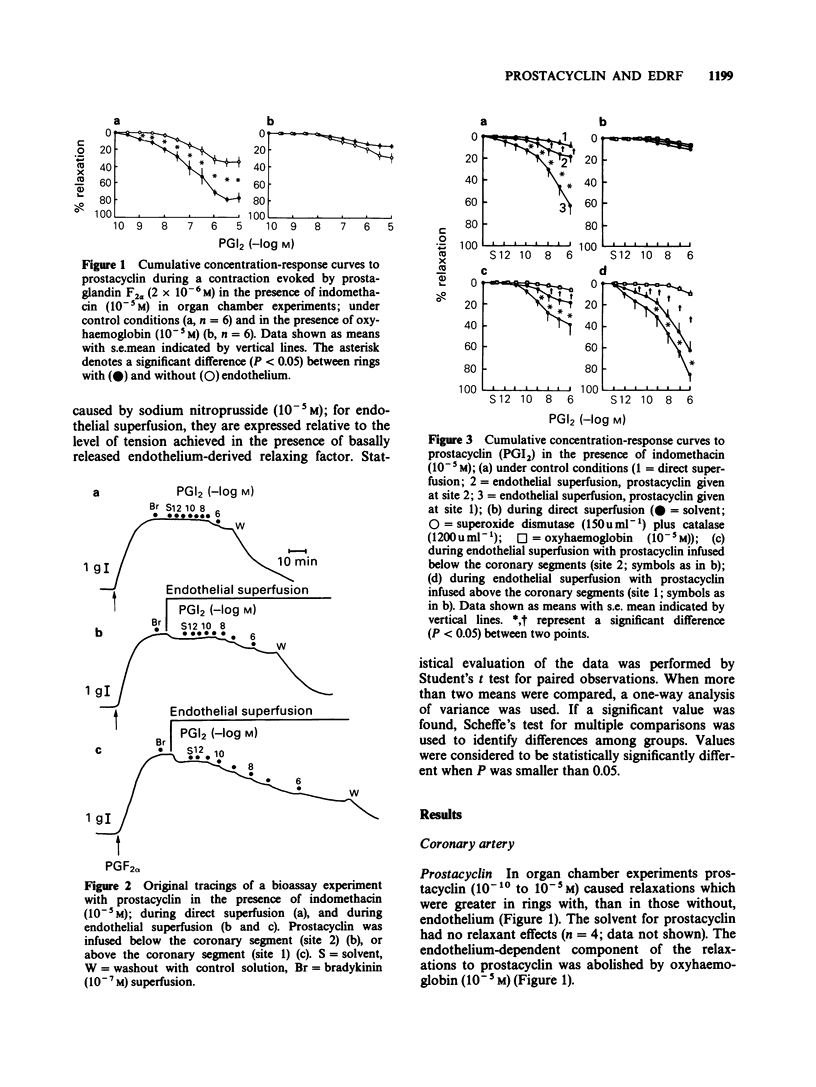
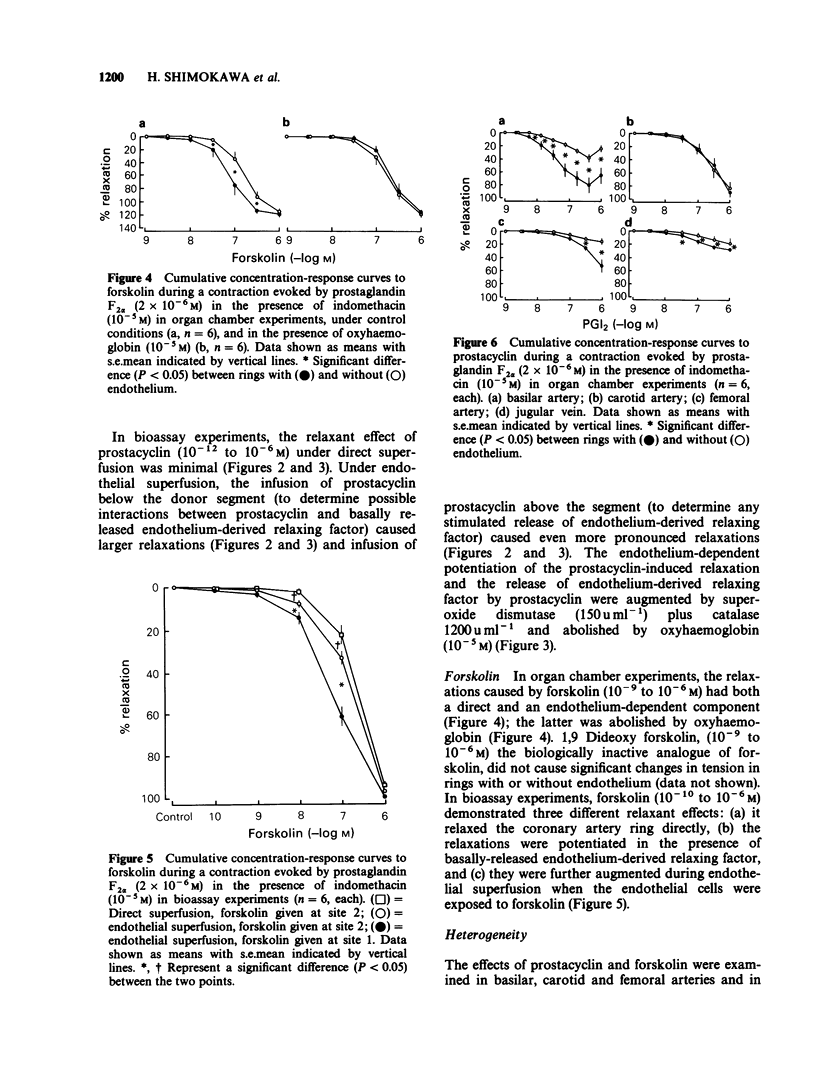
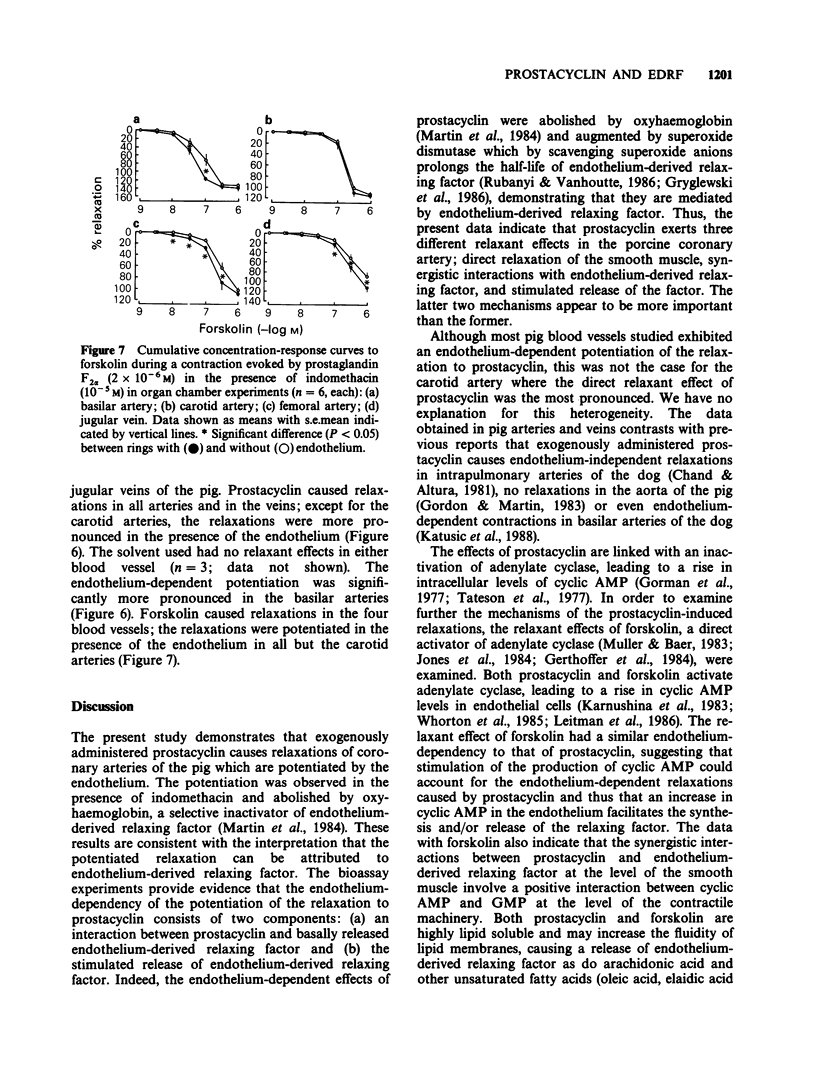
1. The possible interactions between prostacyclin and endothelium-derived relaxing factor were examined, in isolated coronary arteries of the pig treated with indomethacin (10(-5) M). 2. In organ chamber experiments, prostacyclin caused relaxations, which were potentiated in the presence of the endothelium; the potentiation was abolished by oxyhaemoglobin. 3. In bioassay experiments, prostacyclin caused minimal relaxations of bioassay rings without endothelium; these relaxations were potentiated when the bioassay ring was exposed to basally-released endothelium-derived relaxing factor (interaction between prostacyclin and basal endothelium-derived relaxing factor) and further augmented when the endothelial cells were exposed to the prostanoid (stimulated release of endothelium-derived relaxing factor). The endothelium-dependent, but not the direct effects of prostacyclin were augmented by superoxide dismutase plus catalase and abolished by oxyhaemoglobin. 4. Forskolin, a direct activator of adenylate cyclase, caused relaxations of rings without endothelium, which were augmented by the presence of the endothelium. 5. The relaxations induced by prostacyclin or forskolin also had an endothelium-dependent component in basilar and femoral arteries and in jugular veins of the pig. 6. The endothelium-dependent actions of prostacyclin probably reflect activation of adenylate cyclase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma H., Ishikawa M., Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986 Jun;88(2):411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Trogisch G., Bassenge E. The role of endothelium in the control of vascular tone. Basic Res Cardiol. 1985 Sep-Oct;80(5):475–490. doi: 10.1007/BF01907912. [DOI] [PubMed] [Google Scholar]
- Chand N., Altura B. M. Acetylcholine and bradykinin relax intrapulmonary arteries by acting on endothelial cells: role in lung vascular diseases. Science. 1981 Sep 18;213(4514):1376–1379. doi: 10.1126/science.7268440. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Furlong B., Henderson A. H., Lewis M. J., Smith J. A. Endothelium-derived relaxing factor inhibits in vitro platelet aggregation. Br J Pharmacol. 1987 Apr;90(4):687–692. doi: 10.1111/j.1476-5381.1987.tb11221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerthoffer W. T., Trevethick M. A., Murphy R. A. Myosin phosphorylation and cyclic adenosine 3',5'-monophosphate in relaxation of arterial smooth muscle by vasodilators. Circ Res. 1984 Jan;54(1):83–89. doi: 10.1161/01.res.54.1.83. [DOI] [PubMed] [Google Scholar]
- Gordon J. L., Martin W. Stimulation of endothelial prostacyclin production plays no role in endothelium-dependent relaxation of the pig aorta. Br J Pharmacol. 1983 Sep;80(1):179–186. doi: 10.1111/j.1476-5381.1983.tb11064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman R. R., Bunting S., Miller O. V. Modulation of human platelet adenylate cyclase by prostacyclin (PGX). Prostaglandins. 1977 Mar;13(3):377–388. doi: 10.1016/0090-6980(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Jones A. W., Bylund D. B., Forte L. R. cAMP-dependent reduction in membrane fluxes during relaxation of arterial smooth muscle. Am J Physiol. 1984 Feb;246(2 Pt 2):H306–H311. doi: 10.1152/ajpheart.1984.246.2.H306. [DOI] [PubMed] [Google Scholar]
- Karnushina I. L., Spatz M., Bembry J. Cerebral endothelial cell culture. II. Adenylate cyclase response to prostaglandins and their interaction with the adrenergic system. Life Sci. 1983 Mar 28;32(13):1427–1435. doi: 10.1016/0024-3205(83)90907-4. [DOI] [PubMed] [Google Scholar]
- Katusic Z. S., Shepherd J. T., Vanhoutte P. M. Potassium-induced endothelium-dependent rhythmic activity in the canine basilar artery. J Cardiovasc Pharmacol. 1988 Jul;12(1):37–41. doi: 10.1097/00005344-198807000-00005. [DOI] [PubMed] [Google Scholar]
- Leitman D. C., Fiscus R. R., Murad F. Forskolin, phosphodiesterase inhibitors, and cyclic AMP analogs inhibit proliferation of cultured bovine aortic endothelial cells. J Cell Physiol. 1986 May;127(2):237–243. doi: 10.1002/jcp.1041270208. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Muller M. J., Baer H. P. Relaxant effects of forskolin in smooth muscle. Role of cyclic AMP. Naunyn Schmiedebergs Arch Pharmacol. 1983 Feb;322(1):78–82. doi: 10.1007/BF00649356. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Lorenz R. R., Vanhoutte P. M. Bioassay of endothelium-derived relaxing factor(s): inactivation by catecholamines. Am J Physiol. 1985 Jul;249(1 Pt 2):H95–101. doi: 10.1152/ajpheart.1985.249.1.H95. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Romero J. C., Vanhoutte P. M. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986 Jun;250(6 Pt 2):H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Ouabain inhibits endothelium-dependent relaxations to arachidonic acid in canine coronary arteries. J Pharmacol Exp Ther. 1985 Oct;235(1):81–86. [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol. 1986 May;250(5 Pt 2):H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- Shimokawa H., Aarhus L. L., Vanhoutte P. M. Porcine coronary arteries with regenerated endothelium have a reduced endothelium-dependent responsiveness to aggregating platelets and serotonin. Circ Res. 1987 Aug;61(2):256–270. doi: 10.1161/01.res.61.2.256. [DOI] [PubMed] [Google Scholar]
- Shimokawa H., Lam J. Y., Chesebro J. H., Bowie E. J., Vanhoutte P. M. Effects of dietary supplementation with cod-liver oil on endothelium-dependent responses in porcine coronary arteries. Circulation. 1987 Oct;76(4):898–905. doi: 10.1161/01.cir.76.4.898. [DOI] [PubMed] [Google Scholar]
- Tateson J. E., Moncada S., Vane J. R. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977 Mar;13(3):389–397. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Houston D. S. Platelets, endothelium, and vasospasm. Circulation. 1985 Oct;72(4):728–734. doi: 10.1161/01.cir.72.4.728. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Whorton A. R., Collawn J. B., Montgomery M. E., Young S. L., Kent R. S. Arachidonic acid metabolism in cultured aortic endothelial cells. Effect of cAMP and 3-isobutyl-1-methylxanthine. Biochem Pharmacol. 1985 Jan 1;34(1):119–123. doi: 10.1016/0006-2952(85)90109-1. [DOI] [PubMed] [Google Scholar]


