Abstract
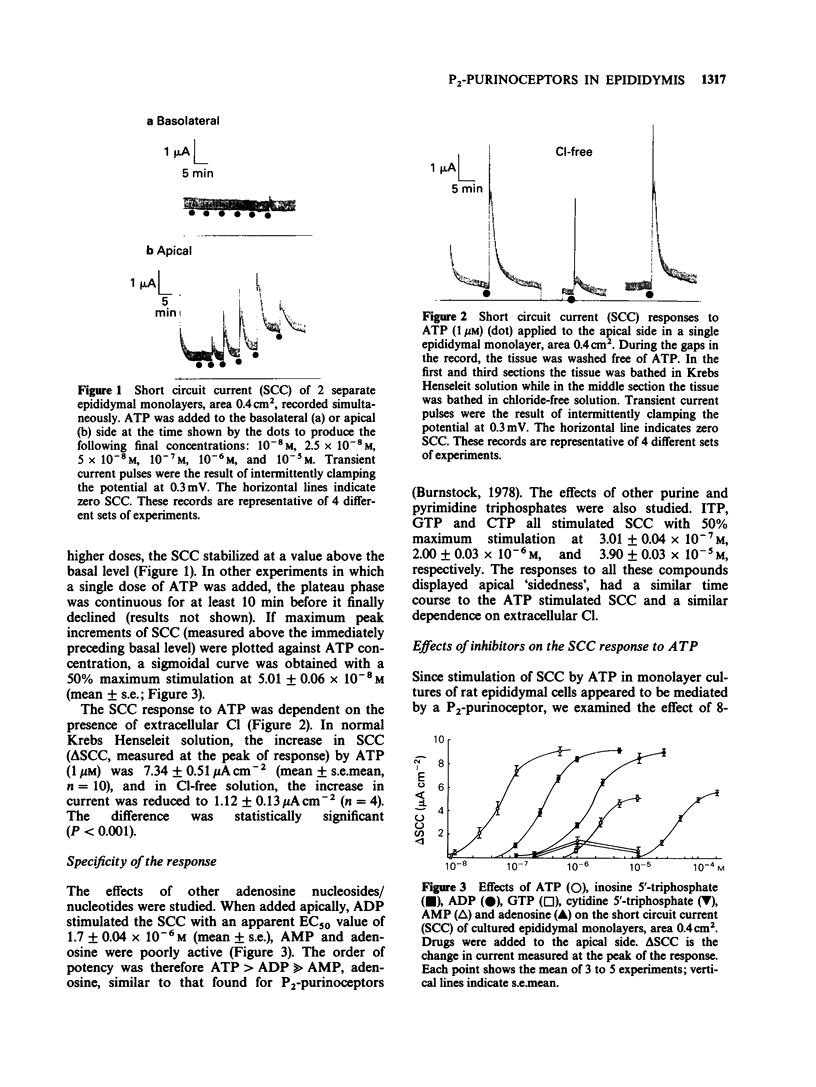
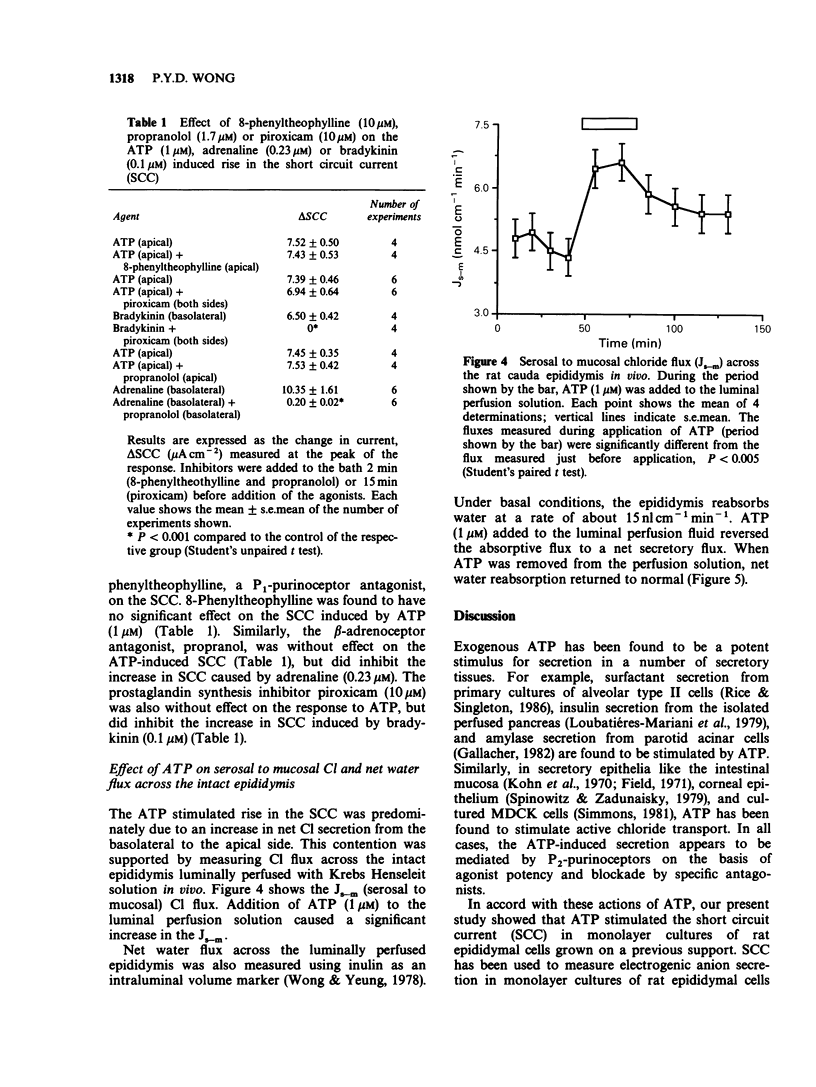
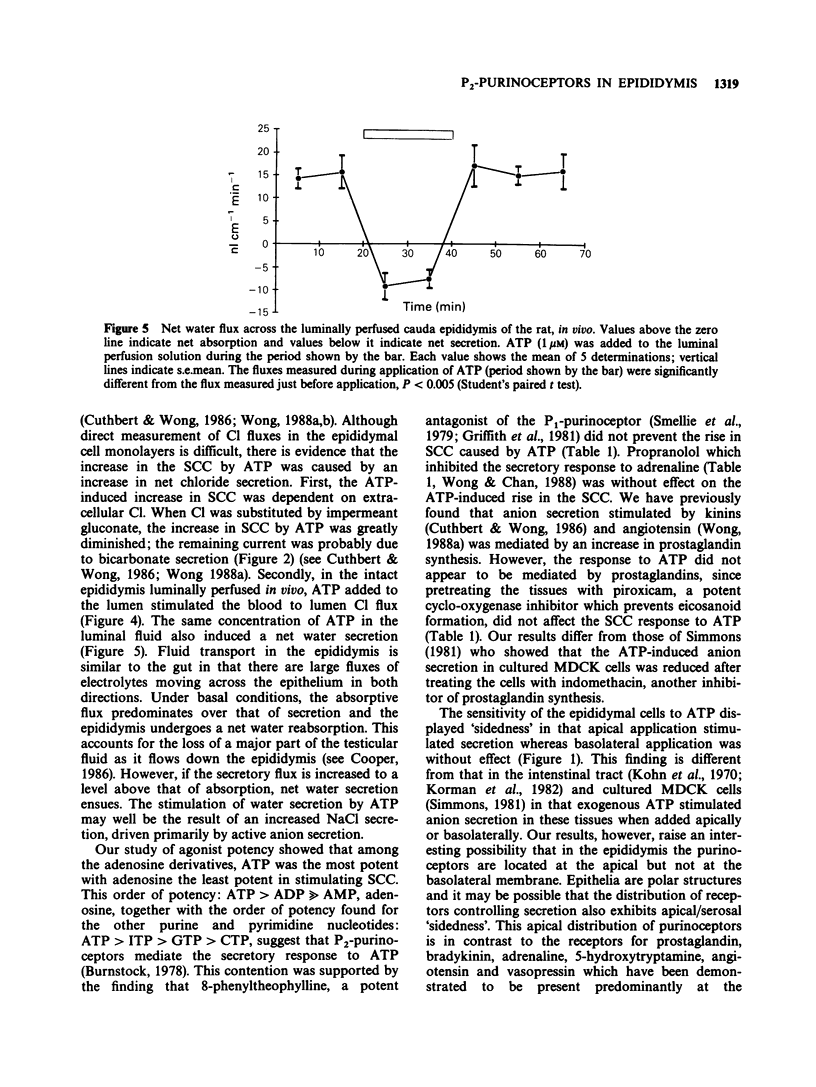
1. Exogenous adenosine triphosphate (ATP) stimulated the short circuit current (SCC) in primary monolayer cultures of rat epididymal cells when added to the apical but not to the basolateral side of the monolayers. Half-maximal stimulation was achieved at 5 x 10(-8) M ATP. 2. The increase in SCC induced by ATP was dependent on the presence of extracellular Cl in the bathing solutions. 3. The effects of other adenosine derivatives, and purine and pyrimidine nucleotides were studied. Their orders of potency in stimulating SCC were: ATP greater than adenosine diphosphate much greater than adenosine monophosphate, adenosine, and ATP greater than inosine triphosphate greater than guanosine triphosphate greater than cytidine triphosphate. These results indicate that ATP interacts with a P2-purinoceptor at the apical membrane of the epididymal cells. 4. The SCC response to ATP was not blocked by 8-phenyltheophylline, a P1-purinoceptor antagonist or by propranolol. Although pretreatment of the cultures with piroxicam abolished the SCC response to bradykinin, it did not affect the response to ATP. This indicates that the SCC response to ATP was not mediated by an increase in the synthesis of prostaglandins. 5. Serosal to mucosal Cl flux (Js-m Cl) and net water flux were measured in the luminally perfused rat epididymis in vivo. ATP (1 microM) added to the luminal perfusion solution caused an increase in Js-m Cl and net water secretion by the epididymal duct. 6. Since spermatozoa contain a high concentration of ATP, it is proposed that ATP released from spermatozoa may affect anion and fluid secretion by the epididymis.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuthbert A. W., Wong P. Y. Electrogenic anion secretion in cultured rat epididymal epithelium. J Physiol. 1986 Sep;378:335–345. doi: 10.1113/jphysiol.1986.sp016222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Ion transport in rabbit ileal mucosa. II. Effects of cyclic 3', 5'-AMP. Am J Physiol. 1971 Oct;221(4):992–997. doi: 10.1152/ajplegacy.1971.221.4.992. [DOI] [PubMed] [Google Scholar]
- Gallacher D. V. Are there purinergic receptors on parotid acinar cells? Nature. 1982 Mar 4;296(5852):83–86. doi: 10.1038/296083a0. [DOI] [PubMed] [Google Scholar]
- Griffith S. G., Meghji P., Moody C. J., Burnstock G. 8-phenyltheophylline: a potent P1-purinoceptor antagonist. Eur J Pharmacol. 1981 Oct 15;75(1):61–64. doi: 10.1016/0014-2999(81)90346-0. [DOI] [PubMed] [Google Scholar]
- Kohn P. G., Newey H., Smyth D. H. The effect of adenosine triphosphate on the transmural potential in rat small intestine. J Physiol. 1970 May;208(1):203–220. doi: 10.1113/jphysiol.1970.sp009114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman L. Y., Lemp G. F., Jackson M. J., Gardner J. D. Mechanism of action of ATP on intestinal epithelial cells. Cyclic AMP-mediated stimulation of active ion transport. Biochim Biophys Acta. 1982 Sep 13;721(1):47–54. doi: 10.1016/0167-4889(82)90022-2. [DOI] [PubMed] [Google Scholar]
- Loubatieres-Mariani M. M., Chapal J., Lignon F., Valette G. Structural specificity of nucleotides for insulin secretory action from the isolated perfused rat pancreas. Eur J Pharmacol. 1979 Nov 16;59(3-4):277–286. doi: 10.1016/0014-2999(79)90291-7. [DOI] [PubMed] [Google Scholar]
- Rice W. R., Singleton F. M. P2-purinoceptors regulate surfactant secretion from rat isolated alveolar type II cells. Br J Pharmacol. 1986 Nov;89(3):485–491. doi: 10.1111/j.1476-5381.1986.tb11148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons N. L. Identification of a purine (P2) receptor linked to ion transport in a cultured renal (MDCK) epithelium. Br J Pharmacol. 1981 Jun;73(2):379–384. doi: 10.1111/j.1476-5381.1981.tb10432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smellie F. W., Davis C. W., Daly J. W., Wells J. N. Alkylxanthines: inhibition of adenosine-elicited accumulation of cyclic AMP in brain slices and of brain phosphodiesterase activity. Life Sci. 1979 Jun 25;24(26):2475–2482. doi: 10.1016/0024-3205(79)90458-2. [DOI] [PubMed] [Google Scholar]
- Spinowitz B. S., Zadunaisky J. A. Action of adenosine on chloride active transport of isolated frog cornea. Am J Physiol. 1979 Aug;237(2):F121–F127. doi: 10.1152/ajprenal.1979.237.2.F121. [DOI] [PubMed] [Google Scholar]
- Wong P. Y. Inhibition by chloride channel blockers of anion secretion in cultured epididymal epithelium and intact epididymis of rats. Br J Pharmacol. 1988 May;94(1):155–163. doi: 10.1111/j.1476-5381.1988.tb11510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. Y. Mechanism of adrenergic stimulation of anion secretion in cultured rat epididymal epithelium. Am J Physiol. 1988 Jan;254(1 Pt 2):F121–F133. doi: 10.1152/ajprenal.1988.254.1.F121. [DOI] [PubMed] [Google Scholar]
- Wong P. Y., Yeung C. H. Absorptive and secretory functions of the perfused rat cauda epididymidis. J Physiol. 1978 Feb;275:13–26. doi: 10.1113/jphysiol.1978.sp012174. [DOI] [PMC free article] [PubMed] [Google Scholar]


