Abstract
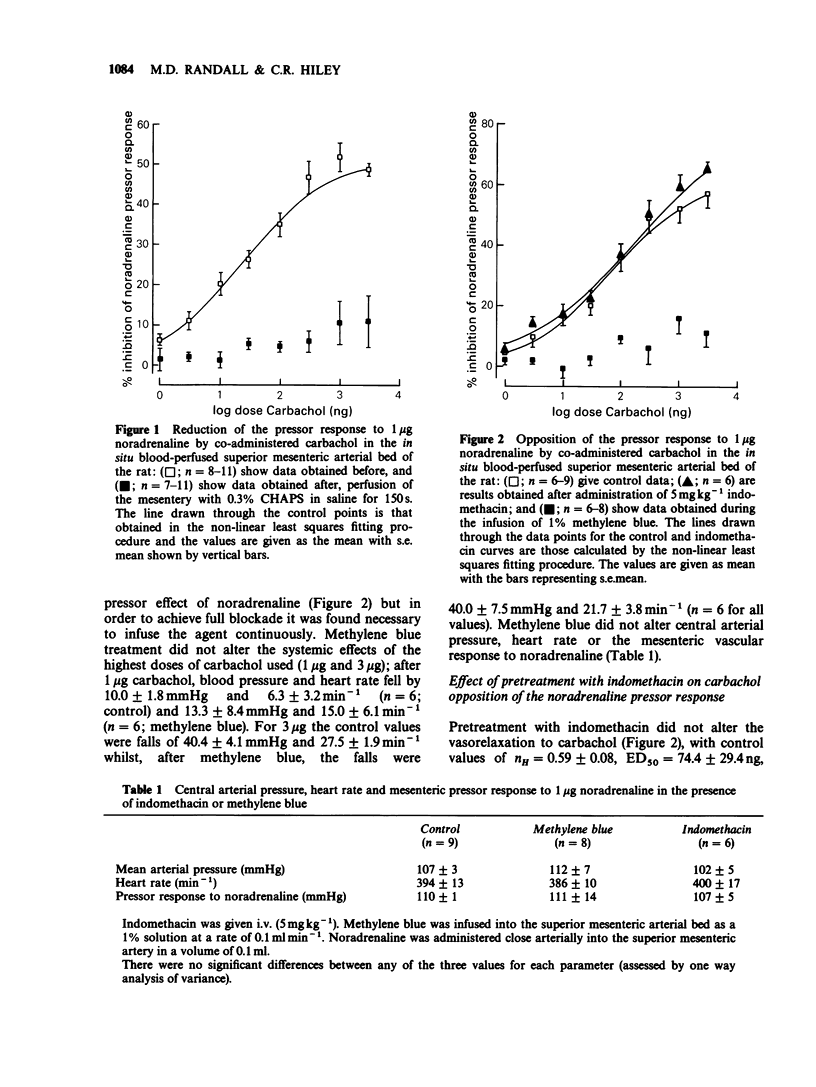
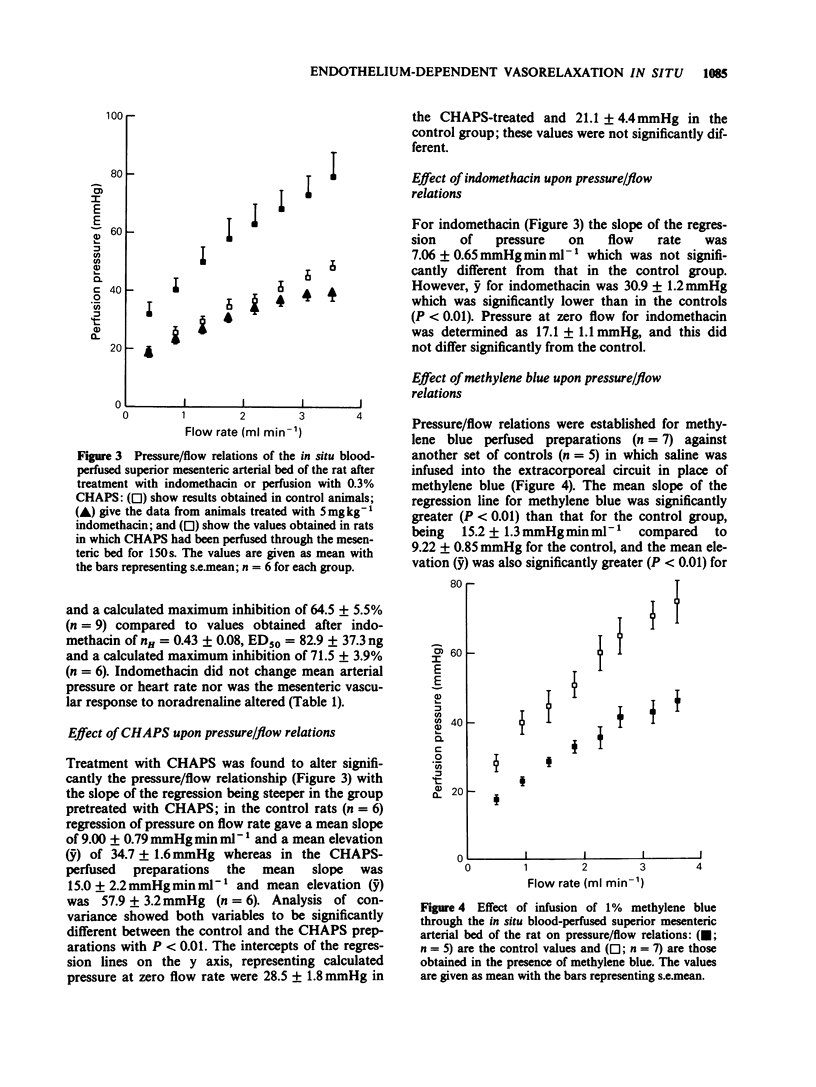
1. The autoperfused superior mesenteric arterial bed of the rat was used to study the opposition by carbachol of pressor responses to noradrenaline and the effects of methylene blue, the detergent CHAPS and indomethacin on pressure/flow relations. 2. Carbachol (1 ng-3 micrograms) reduced the pressor response to 1 microgram noradrenaline in a dose-dependent manner with an ED50 = 25.5 +/- 6.0 ng and a maximum inhibition of 51.8 +/- 1.9%. Perfusion of the mesenteric bed with 0.3% CHAPS in saline for 150s abolished the reduction by carbachol of the noradrenaline pressor response. The effect of carbachol was also abolished by infusion of 1% methylene blue into the mesenteric vascular bed. Indomethacin (5 mg kg-1) was without significant effect on the carbachol opposition of noradrenaline pressor responses. None of the 3 treatments had any effect on the response to 1 microgram noradrenaline. 3. Perfusion with CHAPS before determination of pressure/flow relations, or infusion of methylene blue during their determination, steepened the regression of pressure upon flow to the same extent; at all the flow rates used (0.4-3.54 ml min-1) pressure was greater as a result of the treatment than in control animals. Pretreatment with indomethacin had no effect on pressure/flow relations. 4. It is concluded that carbachol opposition to noradrenaline pressor responses in the blood perfused superior mesenteric arterial bed of the rat shows the characteristics of being mediated by endothelium-dependent relaxing factor (EDRF). Since vascular resistance increases more rapidly than in controls when the endothelium is functionally inhibited by treatment with CHAPS or perfusion with methylene blue, it appears that EDRF has a role in vivo in the modulation of myogenic vascular tone.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aceves J., Mariscal S., Morrison K. E., Young J. M. The binding of doxepin to histamine H1-receptors in guinea-pig and rat brain. Br J Pharmacol. 1985 Feb;84(2):417–424. doi: 10.1111/j.1476-5381.1985.tb12925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus J. A., Campbell G. R., Cocks T. M., Manderson J. A. Vasodilatation by acetylcholine is endothelium-dependent: a study by sonomicrometry in canine femoral artery in vivo. J Physiol. 1983 Nov;344:209–222. doi: 10.1113/jphysiol.1983.sp014934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss W. M. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902 May 28;28(3):220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. P. The antagonism of acetyl choline by methylene blue. J Physiol. 1926 Dec 10;62(2):160–165. doi: 10.1113/jphysiol.1926.sp002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiman P. C., Mitchell G. G., Heistad D. D., Armstrong M. L., Harrison D. G. Atherosclerosis impairs endothelium-dependent vascular relaxation to acetylcholine and thrombin in primates. Circ Res. 1986 Jun;58(6):783–789. doi: 10.1161/01.res.58.6.783. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Davies R. L., Harrison T. J., Evans K. T. EDRF coordinates the behaviour of vascular resistance vessels. Nature. 1987 Oct 1;329(6138):442–445. doi: 10.1038/329442a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Henderson A. H., Edwards D. H., Lewis M. J. Isolated perfused rabbit coronary artery and aortic strip preparations: the role of endothelium-derived relaxant factor. J Physiol. 1984 Jun;351:13–24. doi: 10.1113/jphysiol.1984.sp015228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter C. A., Gruetter D. Y., Lyon J. E., Kadowitz P. J., Ignarro L. J. Relationship between cyclic guanosine 3':5'-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Pharmacol Exp Ther. 1981 Oct;219(1):181–186. [PubMed] [Google Scholar]
- Hiley C. R., Nichols A. J., Wilson A. C. Effects of phenobarbitone and 6-methylprednisolone pretreatment on pressure/flow relations in the superior mesenteric and iliac arterial beds of the rat. J Pharm Pharmacol. 1985 Mar;37(3):164–169. doi: 10.1111/j.2042-7158.1985.tb05033.x. [DOI] [PubMed] [Google Scholar]
- Holzmann S. Endothelium-induced relaxation by acetylcholine associated with larger rises in cyclic GMP in coronary arterial strips. J Cyclic Nucleotide Res. 1982;8(6):409–419. [PubMed] [Google Scholar]
- Jackson E. K., Campbell W. B. The in situ blood perfused rat mesentery; a model for assessing modulation of adrenergic neurotransmission. Eur J Pharmacol. 1980 Aug 29;66(2-3):217–224. doi: 10.1016/0014-2999(80)90145-4. [DOI] [PubMed] [Google Scholar]
- Kaiser L., Hull S. S., Jr, Sparks H. V., Jr Methylene blue and ETYA block flow-dependent dilation in canine femoral artery. Am J Physiol. 1986 Jun;250(6 Pt 2):H974–H981. doi: 10.1152/ajpheart.1986.250.6.H974. [DOI] [PubMed] [Google Scholar]
- Lansman J. B., Hallam T. J., Rink T. J. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? 1987 Feb 26-Mar 4Nature. 325(6107):811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Miller R. C., Mony M., Schini V., Schoeffter P., Stoclet J. C. Endothelial mediated inhibition of contraction and increase in cyclic GMP levels evoked by the alpha-adrenoceptor agonist B-HT 920 in rat isolated aorta. Br J Pharmacol. 1984 Dec;83(4):903–908. doi: 10.1111/j.1476-5381.1984.tb16530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen S. P., Clapham D. E., Davies P. F. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988 Jan 14;331(6152):168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Romero J. C., Vanhoutte P. M. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986 Jun;250(6 Pt 2):H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Smiesko V., Kozík J., Dolezel S. Role of endothelium in the control of arterial diameter by blood flow. Blood Vessels. 1985;22(5):247–251. [PubMed] [Google Scholar]
- Verbeuren T. J., Jordaens F. H., Zonnekeyn L. L., Van Hove C. E., Coene M. C., Herman A. G. Effect of hypercholesterolemia on vascular reactivity in the rabbit. I. Endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986 Apr;58(4):552–564. doi: 10.1161/01.res.58.4.552. [DOI] [PubMed] [Google Scholar]


