Abstract
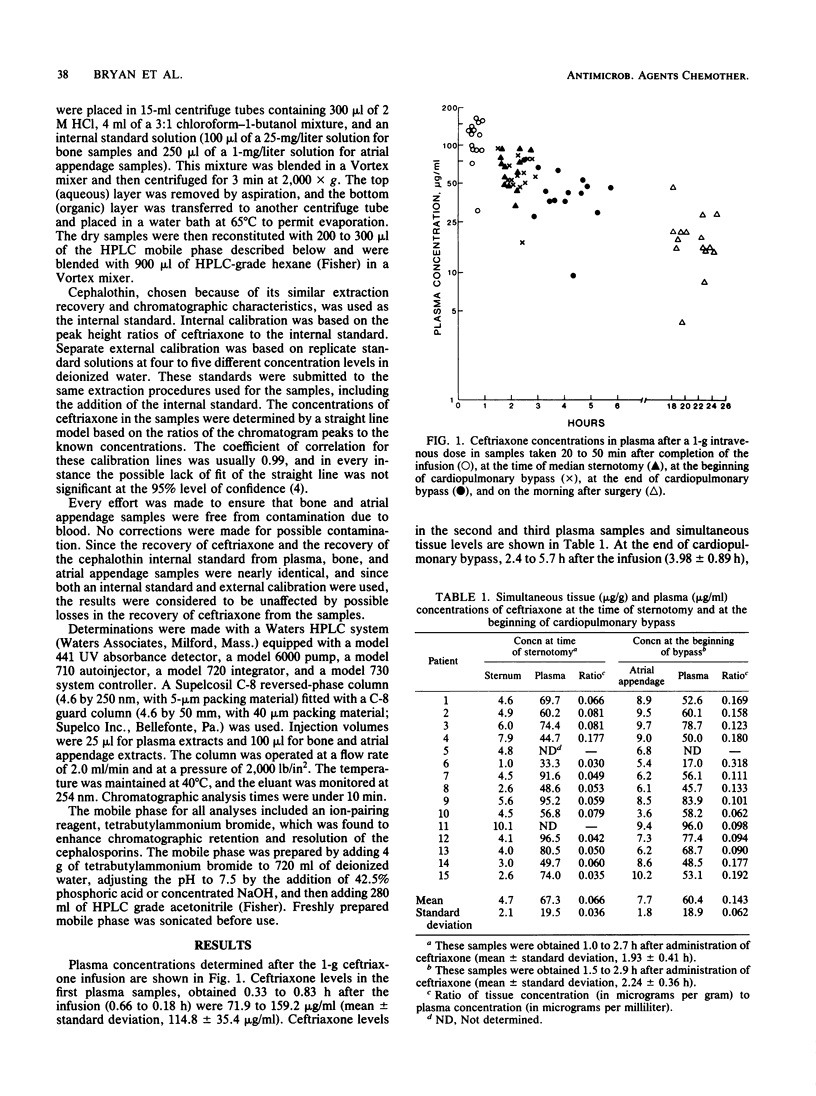
One gram of ceftriaxone was given intravenously to 15 patients approximately 2 h before cardiopulmonary bypass surgery. Ceftriaxone levels in plasma (mean +/- standard deviation) were 60.4 +/- 18.8 micrograms/ml (range, 17.0 to 96.0 micrograms/ml) at the beginning of bypass, 44.2 +/- 16.6 micrograms/ml (range, 9.4 to 78.6 micrograms/ml) at the end of bypass, and 19.6 +/- 9.6 micrograms/ml (range, 4.2 to 47.1 micrograms/ml) the following morning, 18.1 to 24.7 h after infusion of ceftriaxone. Concentrations in the sternal bone were 4.7 +/- 2.1 micrograms/g (range, 1.0 to 10.1 micrograms/g; tissue-to-plasma ratios, 0.066 +/- 0.036). Concentrations in the atrial appendage were 7.7 +/- 1.8 microgram/g (range, 3.6 to 10.2 micrograms/g; tissue-to-plasma ratios, 0.143 +/- 0.062). These data suggest that a single dose of ceftriaxone might be useful for prevention of infection due to susceptible pathogens.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bor D. H., Rose R. M., Modlin J. F., Weintraub R., Friedland G. H. Mediastinitis after cardiovascular surgery. Rev Infect Dis. 1983 Sep-Oct;5(5):885–897. doi: 10.1093/clinids/5.5.885. [DOI] [PubMed] [Google Scholar]
- Brisson A. M., Fourtillan J. B. Determination of cephalosporins in biological material by reversed-phase liquid column chromatography. J Chromatogr. 1981 May 8;223(2):393–399. doi: 10.1016/s0378-4347(00)80112-7. [DOI] [PubMed] [Google Scholar]
- Bryan C. S., Smith C. W., Jr, Sutton J. P., Allen W. B., Blanding R., Gangemi J. D. Comparison of cefamandole and cefazolin during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983 Aug;86(2):222–225. [PubMed] [Google Scholar]
- Deming S. N., Morgan S. L. The use of linear models and matrix least squares in clinical chemistry. Clin Chem. 1979 Jun;25(6):840–855. [PubMed] [Google Scholar]
- Fass R. J. Comparative in vitro activities of third-generation cephalosporins. Arch Intern Med. 1983 Sep;143(9):1743–1745. [PubMed] [Google Scholar]
- Gnann J. W., Jr, Goetter W. E., Elliott A. M., Cobbs C. G. Ceftriaxone: in vitro studies and clinical evaluation. Antimicrob Agents Chemother. 1982 Jul;22(1):1–9. doi: 10.1128/aac.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Meropol N. J., Fu K. P. Antibacterial activity of ceftriaxone (Ro 13-9904), a beta-lactamase-stable cephalosporin. Antimicrob Agents Chemother. 1981 Mar;19(3):414–423. doi: 10.1128/aac.19.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker F. B., Jr, Greiner-Hayes C., Tomar R. H., Markowitz A. H., Bove E. L., Marvasti M. A. Bacteremia following prosthetic valve replacement. Ann Surg. 1983 Feb;197(2):147–151. doi: 10.1097/00000658-198302000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel I. H., Chen S., Parsonnet M., Hackman M. R., Brooks M. A., Konikoff J., Kaplan S. A. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother. 1981 Nov;20(5):634–641. doi: 10.1128/aac.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells F. C., Newsom S. W., Rowlands C. Wound infection in cardiothoracic surgery. Lancet. 1983 May 28;1(8335):1209–1210. doi: 10.1016/s0140-6736(83)92479-0. [DOI] [PubMed] [Google Scholar]


