Abstract
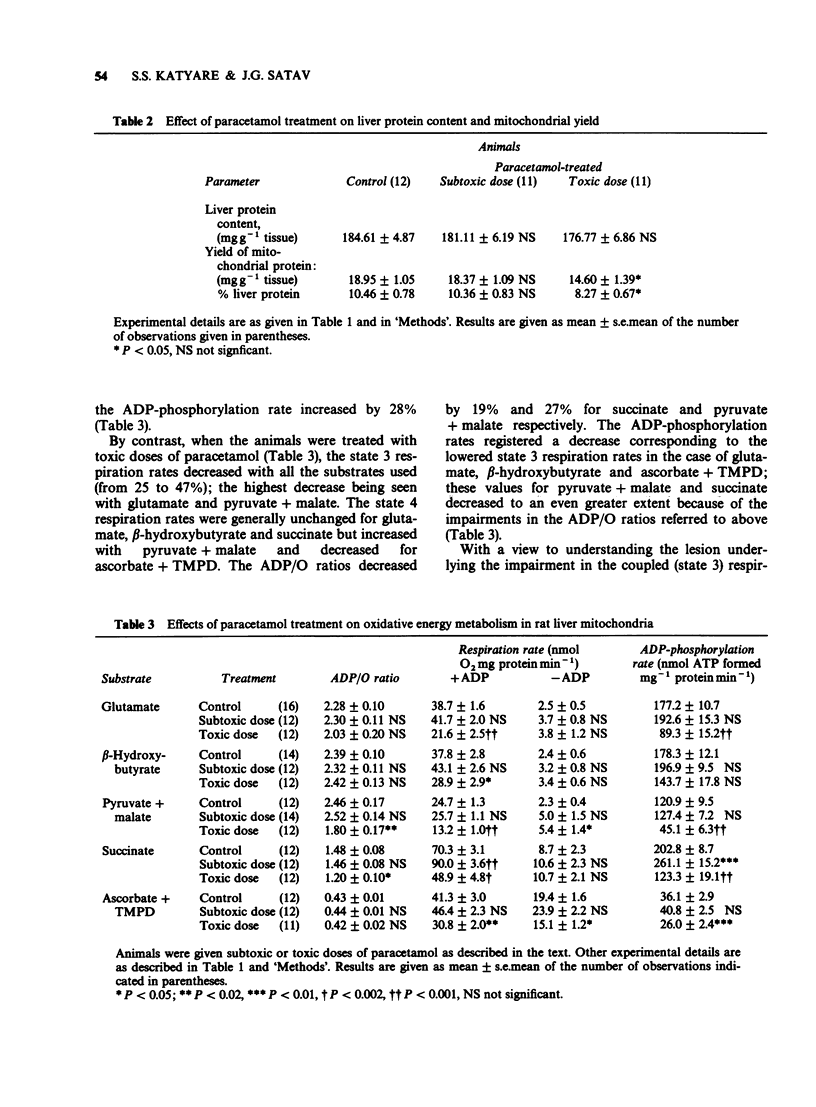
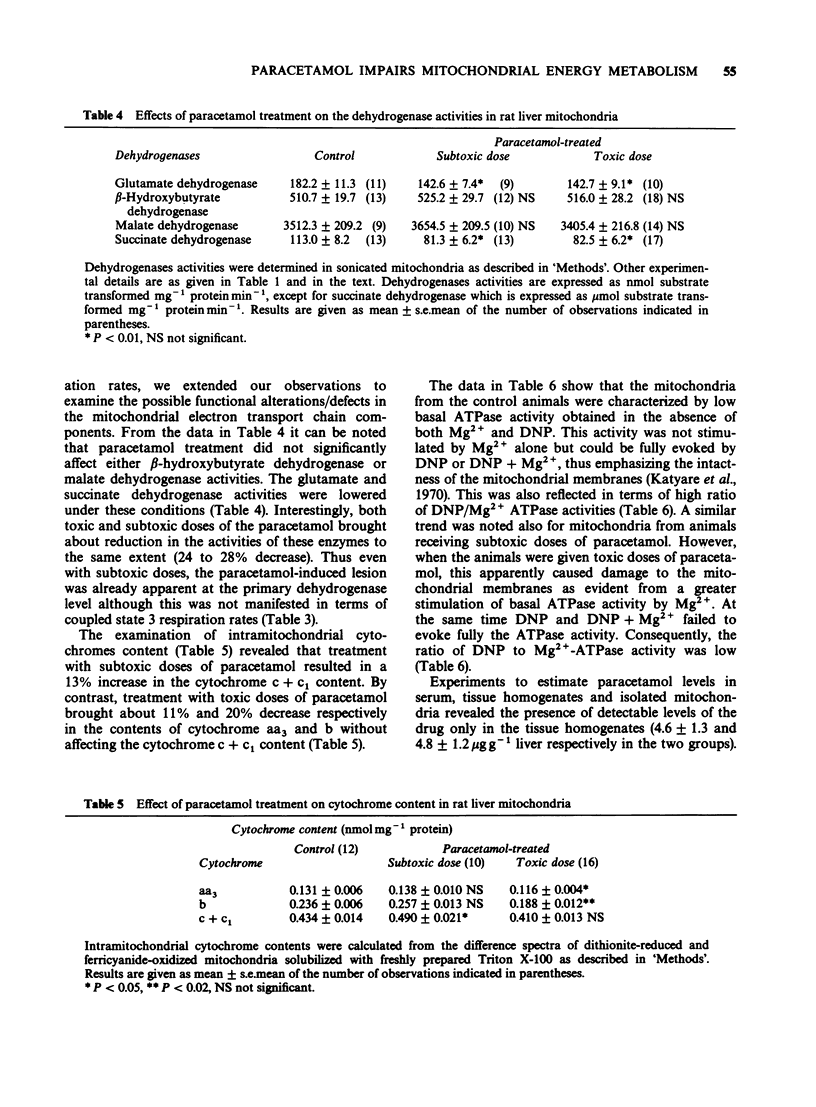
1. Effects of paracetamol treatment in vivo at subtoxic (375 mg kg-1 body weight) and toxic (750 mg kg-1 body weight) doses on energy metabolism in rat liver mitochondria were examined. 2. Paracetamol treatment resulted in a significant loss in body weights without affecting the liver protein contents. Toxic doses, however, resulted in 21% decrease in the yield of mitochondrial proteins. 3. Subtoxic doses of paracetamol did not, in general, affect the respiratory parameters in the liver mitochondria except in the case of succinate where both the state 3 respiration and the ADP-phosphorylation rates increased by 28%. 4. Toxic doses of paracetamol caused 25 to 47% decrease in the state 3 respiration rates depending on the substrate used. ADP/O ratios also decreased significantly with pyruvate + malate and succinate as the substrates. Consequently, ADP-phosphorylation was impaired significantly from 20 to 63%. 5. Subtoxic doses of paracetamol resulted in increased contents of cytochrome c + c1 while the toxic doses caused lowering of the cytochromes aa3 and b contents. 6. Glutamate and succinate dehydrogenase activities decreased in both the experimental groups while Mg2+-ATPase activity was impaired only after toxic dose-treatment. 7. The results show that toxic doses of paracetamol result in impaired energy coupling in the liver mitochondria. Effects of subtoxic doses were also demonstrable in terms of impaired dehydrogenases activities.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breen K. J., Bury R. W., Desmond P. V., Forge B. H., Mashford M. L., Whelan G. Paracetamol self-poisoning: diagnosis, management, and outcome. Med J Aust. 1982 Jan 23;1(2):77–79. doi: 10.5694/j.1326-5377.1982.tb132164.x. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- Cobden I., Record C. O., Ward M. K., Kerr D. N. Paracetamol-induced acute renal failure in the absence of fulminant liver damage. Br Med J (Clin Res Ed) 1982 Jan 2;284(6308):21–22. doi: 10.1136/bmj.284.6308.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M. F., Dixon B., Aparicio S. R., Loney D. P. Experimental paracetamol-induced hepatic necrosis: a light- and electron-microscope, and histochemical study. J Pathol. 1975 May;116(1):17–29. doi: 10.1002/path.1711160104. [DOI] [PubMed] [Google Scholar]
- Ferreira J., Gil L. Nutritional effects on mitochondrial bioenergetics. Alterations in oxidative phosphorylation by rat liver mitochondria. Biochem J. 1984 Feb 15;218(1):61–67. doi: 10.1042/bj2180061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. D., Fischer L. J. Age- and sex-related differences in acetaminophen metabolism in the rat. Life Sci. 1981 Dec 7;29(23):2421–2428. doi: 10.1016/0024-3205(81)90479-3. [DOI] [PubMed] [Google Scholar]
- Hinson J. A., Mays J. B., Cameron A. M. Acetaminophen-induced hepatic glycogen depletion and hyperglycemia in mice. Biochem Pharmacol. 1983 Jul 1;32(13):1979–1988. doi: 10.1016/0006-2952(83)90415-x. [DOI] [PubMed] [Google Scholar]
- Hinson J. A., Pohl L. R., Monks T. J., Gillette J. R. Acetaminophen-induced hepatotoxicity. Life Sci. 1981 Jul 13;29(2):107–116. doi: 10.1016/0024-3205(81)90278-2. [DOI] [PubMed] [Google Scholar]
- Katyare S. S., Fatterpaker P., Sreenivasan A. Effect of 2, 4-dinitrophenol (DNP) on oxidative phosphorylation in rat liver mitochondria. Arch Biochem Biophys. 1971 May;144(1):209–215. doi: 10.1016/0003-9861(71)90470-x. [DOI] [PubMed] [Google Scholar]
- Katyare S. S., Fatterpaker P., Sreenivasan A. Heterogeneity of rat liver mitochondrial fractions and the effect of tri-iodothyronine on their protein turnover. Biochem J. 1970 Jun;118(1):111–121. doi: 10.1042/bj1180111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katyare S. S., Rajan R. R. Enhanced oxidative phosphorylation in rat liver mitochondria following prolonged in vivo treatment with imipramine. Br J Pharmacol. 1988 Nov;95(3):914–922. doi: 10.1111/j.1476-5381.1988.tb11721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain C. J. Late presentation of acetaminophen hepatotoxicity. An unresolved problem. Dig Dis Sci. 1982 Apr;27(4):375–376. doi: 10.1007/BF01296761. [DOI] [PubMed] [Google Scholar]
- Meola J. M. Emergency determination of acetaminophen. Clin Chem. 1978 Sep;24(9):1642–1643. [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Davis D. C., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973 Oct;187(1):185–194. [PubMed] [Google Scholar]
- Miyahara M., Utsumi K., Deamer D. W. Selective interaction of D-beta-hydroxybutyrate dehydrogenase with intracellular membranes. Biochim Biophys Acta. 1981 Feb 20;641(1):222–231. doi: 10.1016/0005-2736(81)90586-1. [DOI] [PubMed] [Google Scholar]
- Newton J. F., Yoshimoto M., Bernstein J., Rush G. F., Hook J. B. Acetaminophen nephrotoxicity in the rat. II. Strain differences in nephrotoxicity and metabolism of p-aminophenol, a metabolite of acetaminophen. Toxicol Appl Pharmacol. 1983 Jun 30;69(2):307–318. doi: 10.1016/0041-008x(83)90312-5. [DOI] [PubMed] [Google Scholar]
- Porter K. E., Dawson A. G. Inhibition of respiration and gluconeogenesis by paracetamol in rat kidney preparations. Biochem Pharmacol. 1979 Oct 15;28(20):3057–3062. doi: 10.1016/0006-2952(79)90613-0. [DOI] [PubMed] [Google Scholar]
- Satav J. G., Katyare S. S. Effect of experimental thyrotoxicosis on oxidative phosphorylation in rat liver, kidney and brain mitochondria. Mol Cell Endocrinol. 1982 Oct;28(2):173–189. doi: 10.1016/0303-7207(82)90030-2. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Swanson M. B., Walters M. I. Rapid colorimetric assay for acetaminophen without salicylate or phenylephrine interference. Clin Chem. 1982 May;28(5):1171–1173. [PubMed] [Google Scholar]
- Walker R. M., Massey T. E., McElligott T. F., Racz W. J. Acetaminophen toxicity in fed and fasted mice. Can J Physiol Pharmacol. 1982 Mar;60(3):399–404. doi: 10.1139/y82-058. [DOI] [PubMed] [Google Scholar]


