Abstract
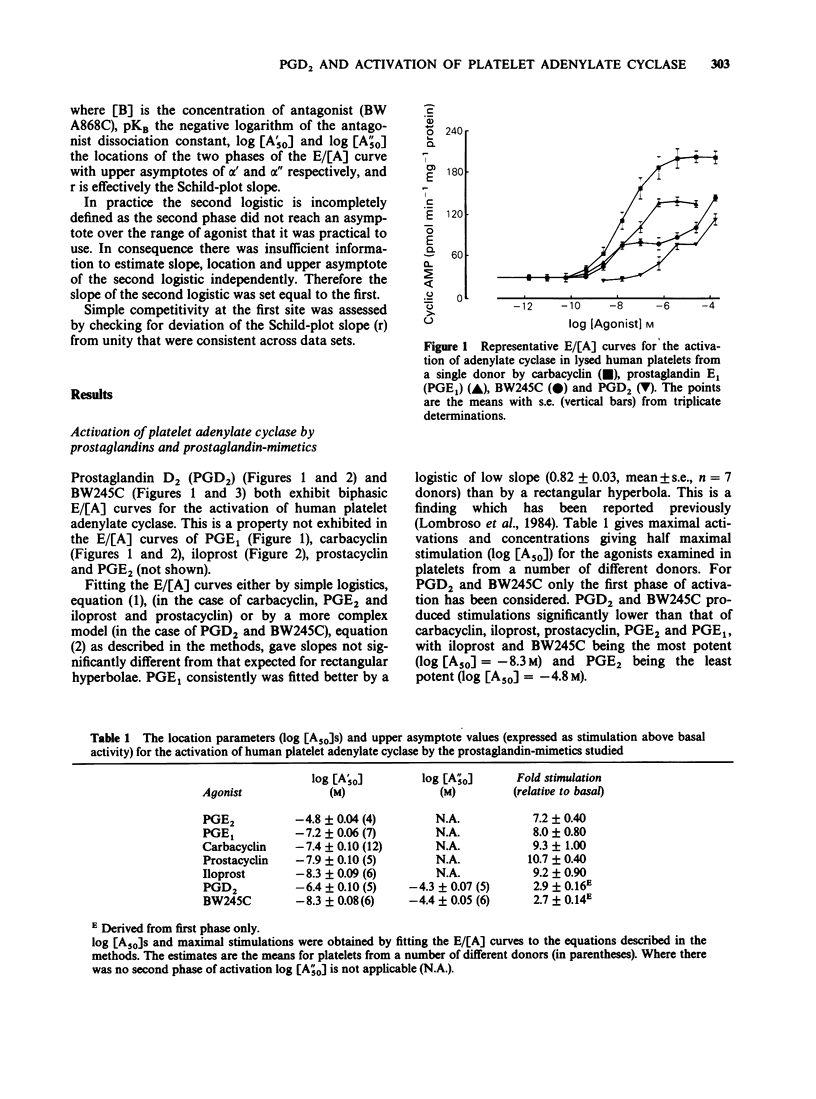
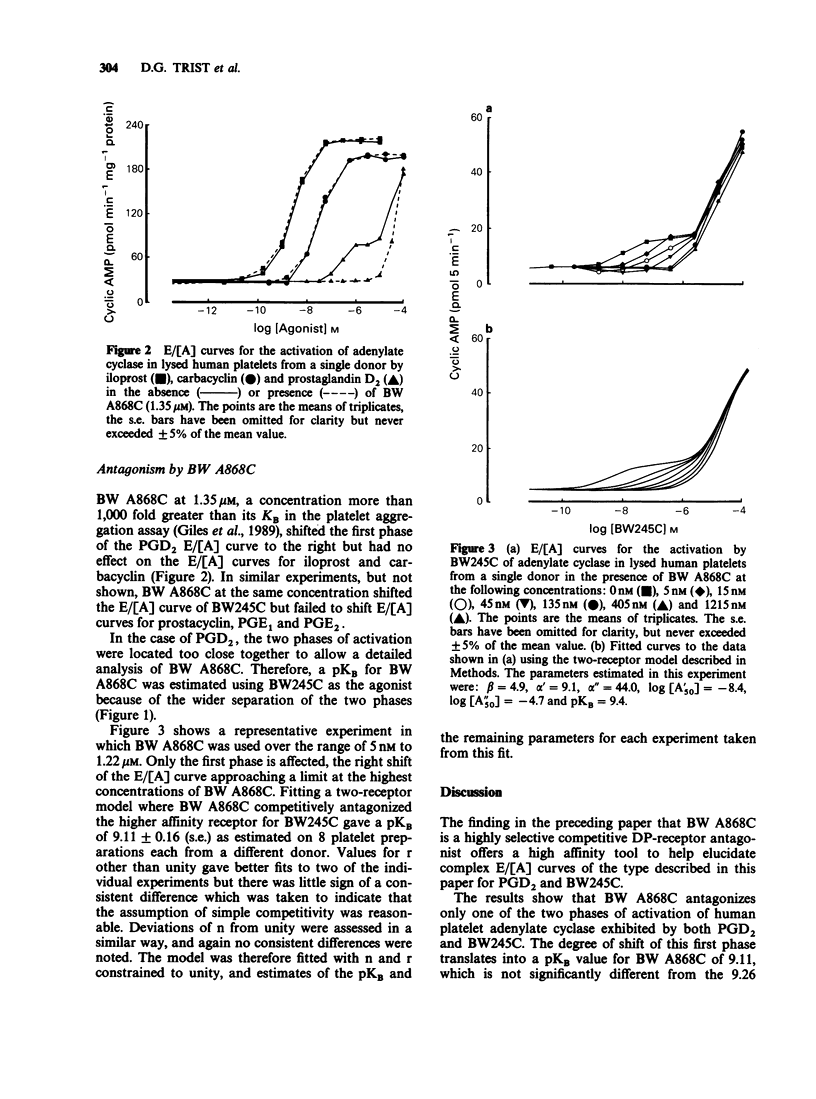
1. In glycerol-lysed human platelets, prostaglandin D2 (PGD2) and the hydantoin BW245C both activate adenylate cyclase in a biphasic manner. These activations are qualitatively different from those of carbacyclin, iloprost and prostaglandin E2 (PGE2) whose E/[A] curves can be adequately described by rectangular hyperbolae. 2. Prostaglandin E1 (PGE1) had E/[A] curves of slope significantly lower than that expected for a rectangular hyperbolae. 2. Prostaglandin E1 (PGE1) had E/[A] curves of slope significantly lower than that expected for a rectangular hyperbola. 3. The selective PGD2 antagonist BW A868C shifts the first phase of the PGD2 and BW245C E/[A] curves but has no effect on the second phase. 4. Applying a two-receptor model enables a pKB to be derived for BW A868C of 9.11. 5. BW A868C has no effect on carbacyclin, iloprost, prostacyclin, PGE1 and PGE2 at a concentration 1,000 fold that of its KB against PGD2 and BW245C. 6. These results indicate that PGD2 and BW245C are capable of activating adenylate cyclase in human platelets through the DP-receptor and by another mechanism as yet uncharacterized.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dong Y. J., Jones R. L., Wilson N. H. Prostaglandin E receptor subtypes in smooth muscle: agonist activities of stable prostacyclin analogues. Br J Pharmacol. 1986 Jan;87(1):97–107. doi: 10.1111/j.1476-5381.1986.tb10161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles H., Leff P., Bolofo M. L., Kelly M. G., Robertson A. D. The classification of prostaglandin DP-receptors in platelets and vasculature using BW A868C, a novel, selective and potent competitive antagonist. Br J Pharmacol. 1989 Feb;96(2):291–300. doi: 10.1111/j.1476-5381.1989.tb11816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman R. R., Bunting S., Miller O. V. Modulation of human platelet adenylate cyclase by prostacyclin (PGX). Prostaglandins. 1977 Mar;13(3):377–388. doi: 10.1016/0090-6980(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Hall J. M., Strange P. G. The use of a prostacyclin analogue, [3H]iloprost, for studying prostacyclin-binding sites on human platelets and neuronal hybrid cells. Biosci Rep. 1984 Nov;4(11):941–948. doi: 10.1007/BF01116892. [DOI] [PubMed] [Google Scholar]
- Kennedy I., Coleman R. A., Humphrey P. P., Levy G. P., Lumley P. Studies on the characterisation of prostanoid receptors: a proposed classification. Prostaglandins. 1982 Nov;24(5):667–689. doi: 10.1016/0090-6980(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Lombroso M., Nicosia S., Paoletti R., Whittle B. J., Moncada S., Vane J. R. The use of stable prostaglandins to investigate prostacyclin (PGI2)-binding sites and PGI2-sensitive adenylate cyclase in human platelet membranes. Prostaglandins. 1984 Feb;27(2):321–333. doi: 10.1016/0090-6980(84)90083-2. [DOI] [PubMed] [Google Scholar]
- Miller O. V., Gorman R. R. Evidence for distinct prostaglandin I2 and D2 receptors in human platelets. J Pharmacol Exp Ther. 1979 Jul;210(1):134–140. [PubMed] [Google Scholar]
- Miller O. V., Gorman R. R. Modulation of platelet cyclic nucleotide content by PGE1 and the prostaglandin endoperoxide PGG2. J Cyclic Nucleotide Res. 1976;2(2):79–87. [PubMed] [Google Scholar]
- Miller O. V., Johnson R. A., Gorman R. R. Inhibition of PGE1-stimulated cAMP accumulation in human platelets by thromboxane a2. Prostaglandins. 1977 Apr;13(4):599–609. doi: 10.1016/0090-6980(77)90231-3. [DOI] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Siegl A. M., Smith J. B., Silver M. J., Nicolaou K. C., Ahern D. Selective binding site for [3H]prostacyclin on platelets. J Clin Invest. 1979 Feb;63(2):215–220. doi: 10.1172/JCI109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer M. L., Wood A. Regulation of human platelet adenylate cyclase by epinephrine, prostaglandin E1, and guanine nucleotides. Evidence for separate guanine nucleotide sites mediating stimulation and inhibition. J Biol Chem. 1979 Nov 10;254(21):10791–10797. [PubMed] [Google Scholar]
- Town M. H., Casals-Stenzel J., Schillinger E. Pharmacological and cardiovascular properties of a hydantoin derivative, BW 245 C, with high affinity and selectivity for PGD2 receptors. Prostaglandins. 1983 Jan;25(1):13–28. doi: 10.1016/0090-6980(83)90131-4. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Moncada S., Vane J. R. Comparison of the effects of prostacyclin (PGI2), prostaglandin E1 and D2 on platelet aggregation in different species. Prostaglandins. 1978 Sep;16(3):373–388. doi: 10.1016/0090-6980(78)90216-2. [DOI] [PubMed] [Google Scholar]


