Abstract
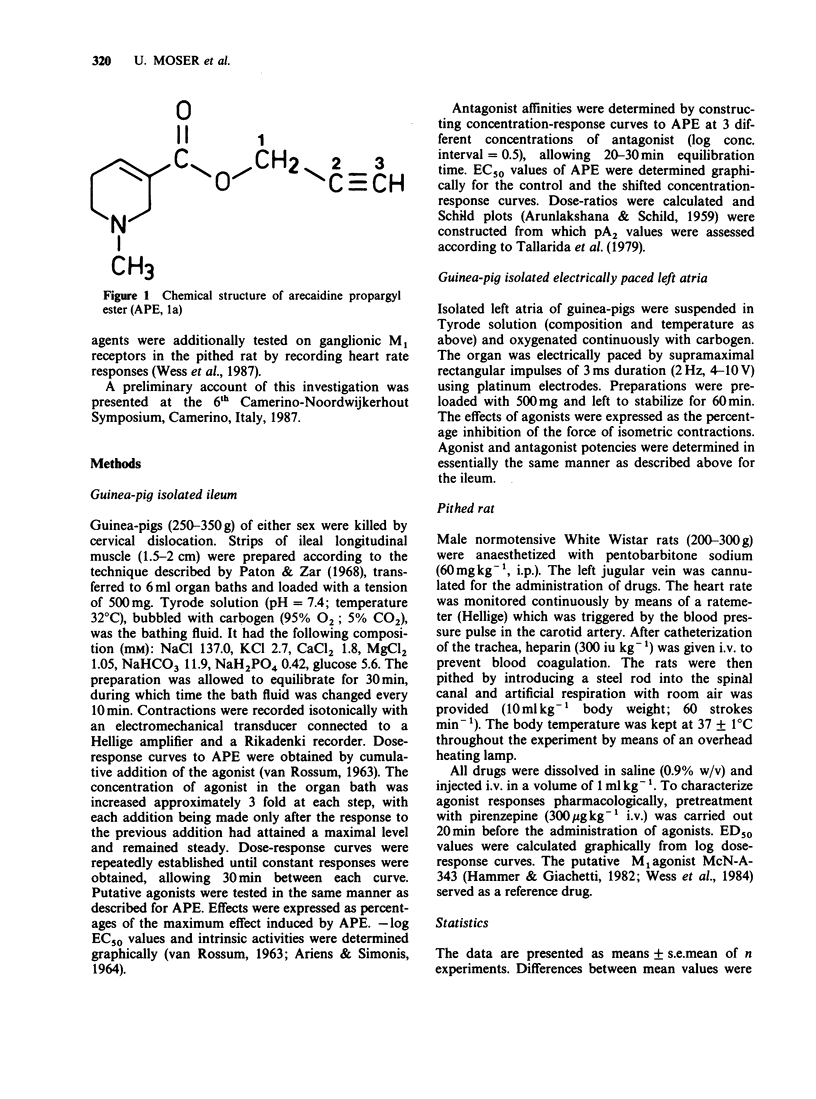
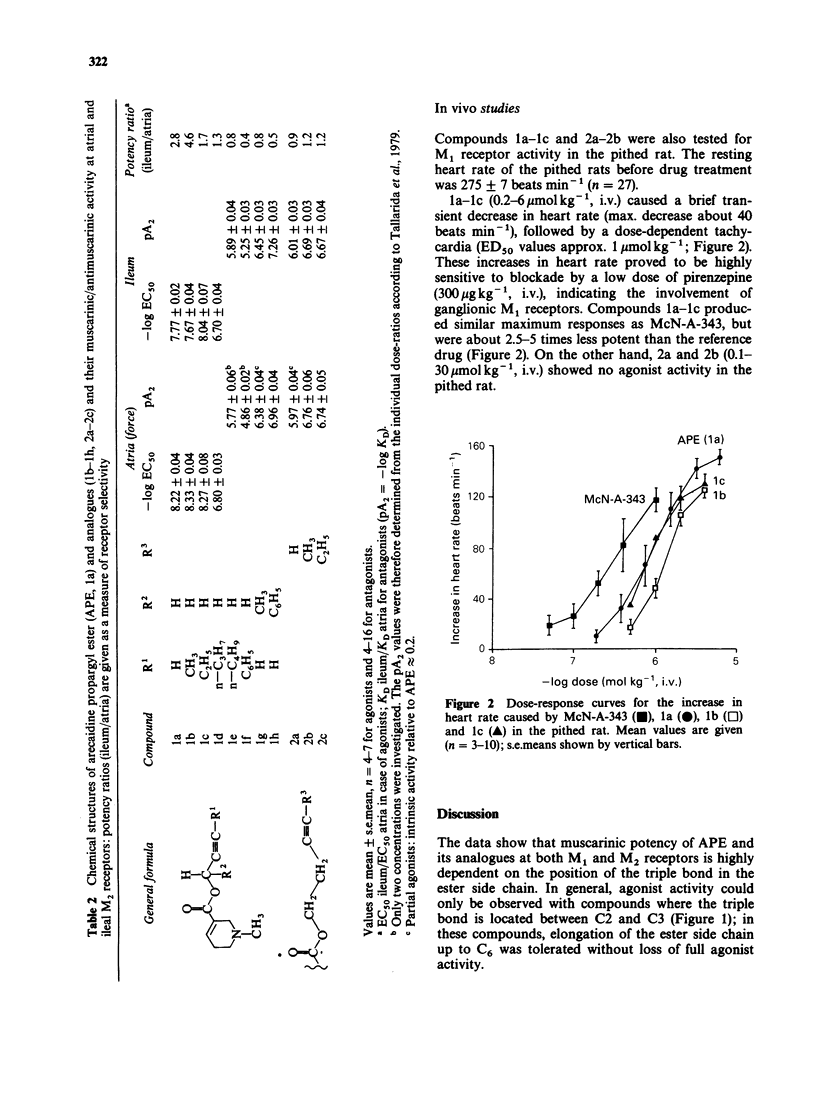
1. The potency of arecaidine propargyl ester (APE) and of several analogues containing a modified ester side chain has been assessed at M1 and M2 muscarinic receptor subtypes. APE was shown to act as a potent agonist at ganglionic M1 receptors in the pithed rat, at M2 receptors in guinea-pig isolated atria (-log EC50 = 8.22) and ileum (-log EC50 = 7.77). 2. The arecaidine 2-butynyl and 2-pentynyl esters were approximately equipotent with APE at M1 and M2 receptors, whereas the 2-hexynyl derivative was found to be less potent than APE in atria (-log EC50 = 6.80) and ileum (-log EC50 = 6.70) by about one order of magnitude. The 2-heptynyl and 3-phenyl propargyl esters exhibited no agonist actions in atria and ileum. 3. Shifting the triple bond from the 2 to the 3 position and introducing a bulky group at position 1 of the ester side chain of APE and analogues resulted in competitive antagonists (pA2 ranging from 4.9 to 7.3). 4. APE and its 2-butynyl analogue showed some agonistic selectivity for cardiac M2 receptors (potency ratio, ileum/atria = 2.8 and 4.6 respectively). All antagonists in this series of compounds were not selective in terms of affinity since their pA2 values at cardiac and ileal M2 receptors were similar (potency ratios, ileum/atria = 0.4 to 1.2).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIUENS E. J., SIMONIS A. M. A MOLECULAR BASIS FOR DRUG ACTION. J Pharm Pharmacol. 1964 Mar;16:137–157. doi: 10.1111/j.2042-7158.1964.tb07437.x. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstutz R., Ringdahl B., Karlén B., Roch M., Jenden D. J. Stereoselectivity of muscarinic receptors in vivo and in vitro for oxotremorine analogues. N-[4-(tertiary amino)-2-butynyl]-5-methyl-2-pyrrolidones. J Med Chem. 1985 Dec;28(12):1760–1765. doi: 10.1021/jm00150a004. [DOI] [PubMed] [Google Scholar]
- Ariens E. J., Simonis A. M. Cholinergic and anticholinergic drugs, do they act on common receptors? Ann N Y Acad Sci. 1967 Oct 31;144(2):842–869. doi: 10.1111/j.1749-6632.1967.tb53815.x. [DOI] [PubMed] [Google Scholar]
- Barlow R. B., Weston-Smith P. The relative potencies of some agonists at M2 muscarinic receptors in guinea-pig ileum, atria and bronchi. Br J Pharmacol. 1985 Jun;85(2):437–440. doi: 10.1111/j.1476-5381.1985.tb08879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doods H. N., Mathy M. J., Davidesko D., van Charldorp K. J., de Jonge A., van Zwieten P. A. Selectivity of muscarinic antagonists in radioligand and in vivo experiments for the putative M1, M2 and M3 receptors. J Pharmacol Exp Ther. 1987 Jul;242(1):257–262. [PubMed] [Google Scholar]
- Eglen R. M., Whiting R. L. Muscarinic receptor subtypes: a critique of the current classification and a proposal for a working nomenclature. J Auton Pharmacol. 1986 Dec;6(4):323–346. doi: 10.1111/j.1474-8673.1986.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Hammer R., Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 1982 Dec 27;31(26):2991–2998. doi: 10.1016/0024-3205(82)90066-2. [DOI] [PubMed] [Google Scholar]
- Mutschler E., Hultzsch K. Uber Struktur-Wirkungs-Beziehungen von ungesättigten Estern des Arecaidins und Dihydroarecaidins. Arzneimittelforschung. 1973 May;23(5):732–737. [PubMed] [Google Scholar]
- Paton W. D., Zar M. A. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol. 1968 Jan;194(1):13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringdahl B., Jenden D. J. Affinity, efficacy, and stereoselectivity of oxotremorine analogues for muscarinic receptors in the isolated guinea pig ileum. Mol Pharmacol. 1983 Jan;23(1):17–25. [PubMed] [Google Scholar]
- Tallarida R. J., Cowan A., Adler M. W. pA2 and receptor differentiation: a statistical analysis of competitive antagonism. Life Sci. 1979 Aug 20;25(8):637–654. doi: 10.1016/0024-3205(79)90505-8. [DOI] [PubMed] [Google Scholar]
- VAN ROSSUM J. M. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther. 1963;143:299–330. [PubMed] [Google Scholar]
- Wess J., Lambrecht G., Moser U., Mutschler E. A comparison of the antimuscarinic effects of pirenzepine and N-methylatropine on ganglionic and vascular muscarinic receptors in the rat. Life Sci. 1984 Jul 30;35(5):553–560. doi: 10.1016/0024-3205(84)90249-2. [DOI] [PubMed] [Google Scholar]
- Wess J., Lambrecht G., Moser U., Mutschler E. Stimulation of ganglionic muscarinic M1 receptors by a series of tertiary arecaidine and isoarecaidine esters in the pithed rat. Eur J Pharmacol. 1987 Jan 28;134(1):61–67. doi: 10.1016/0014-2999(87)90131-2. [DOI] [PubMed] [Google Scholar]


