Abstract
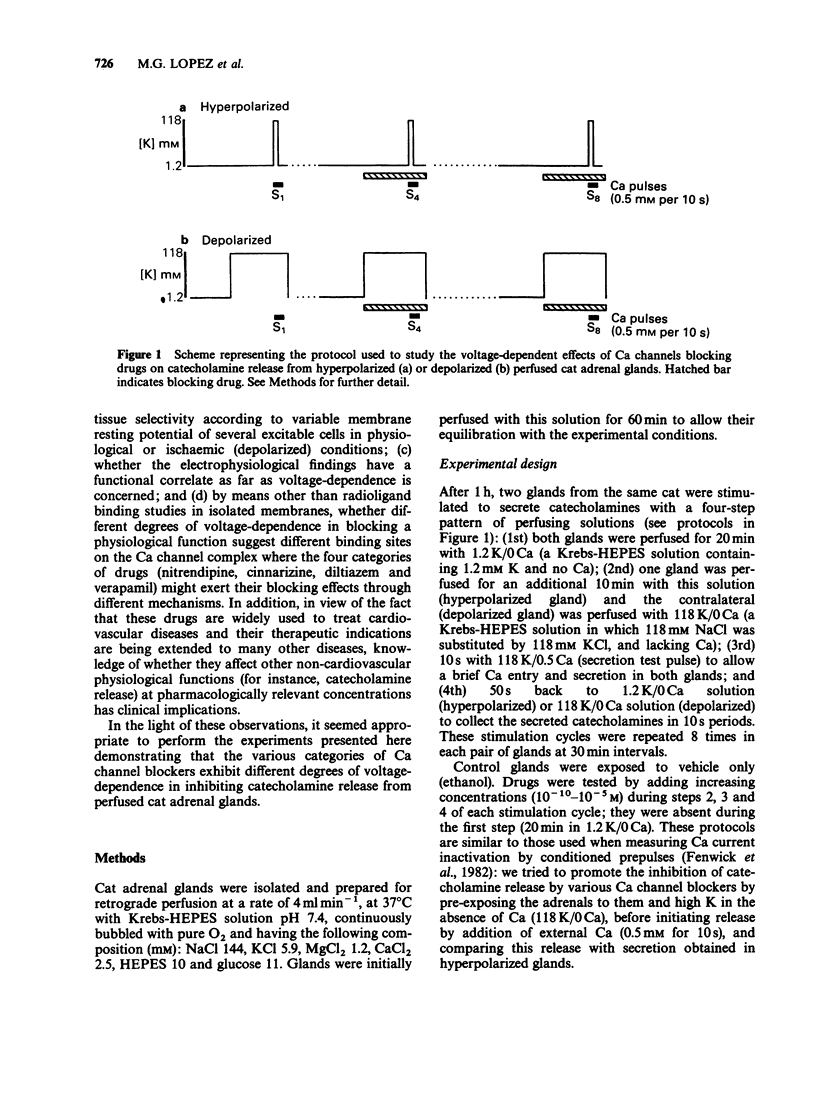
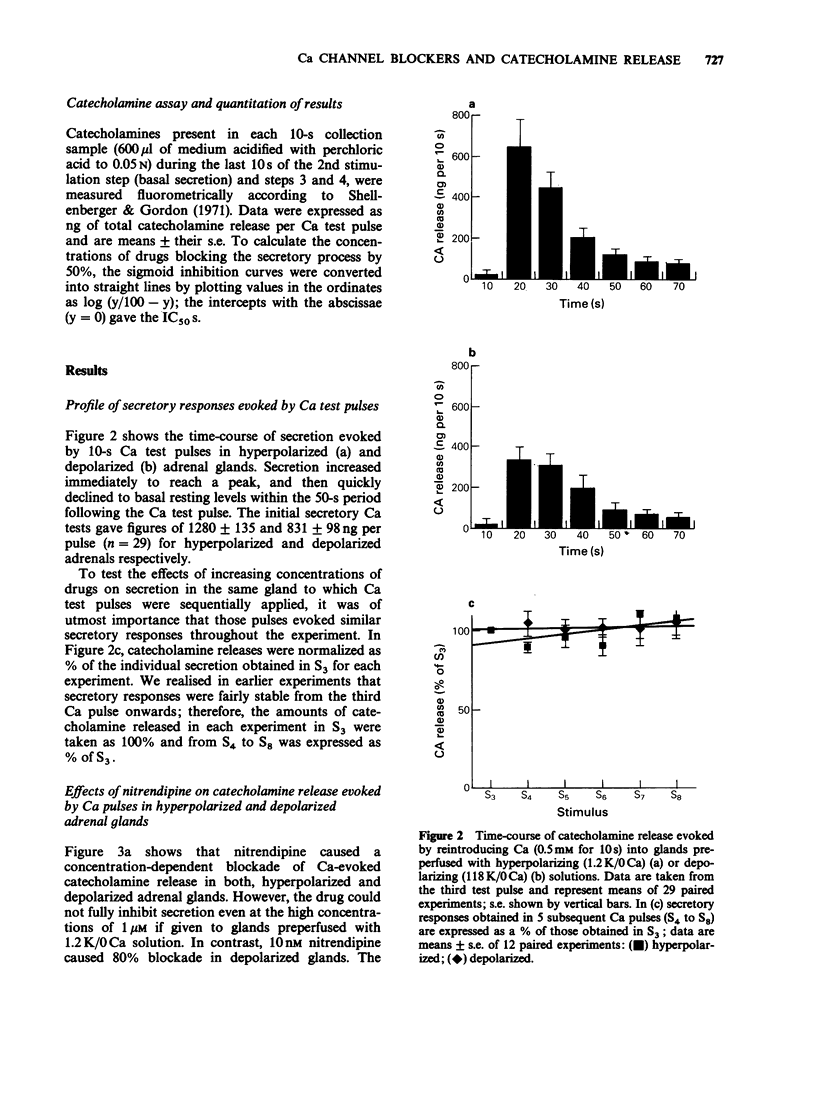
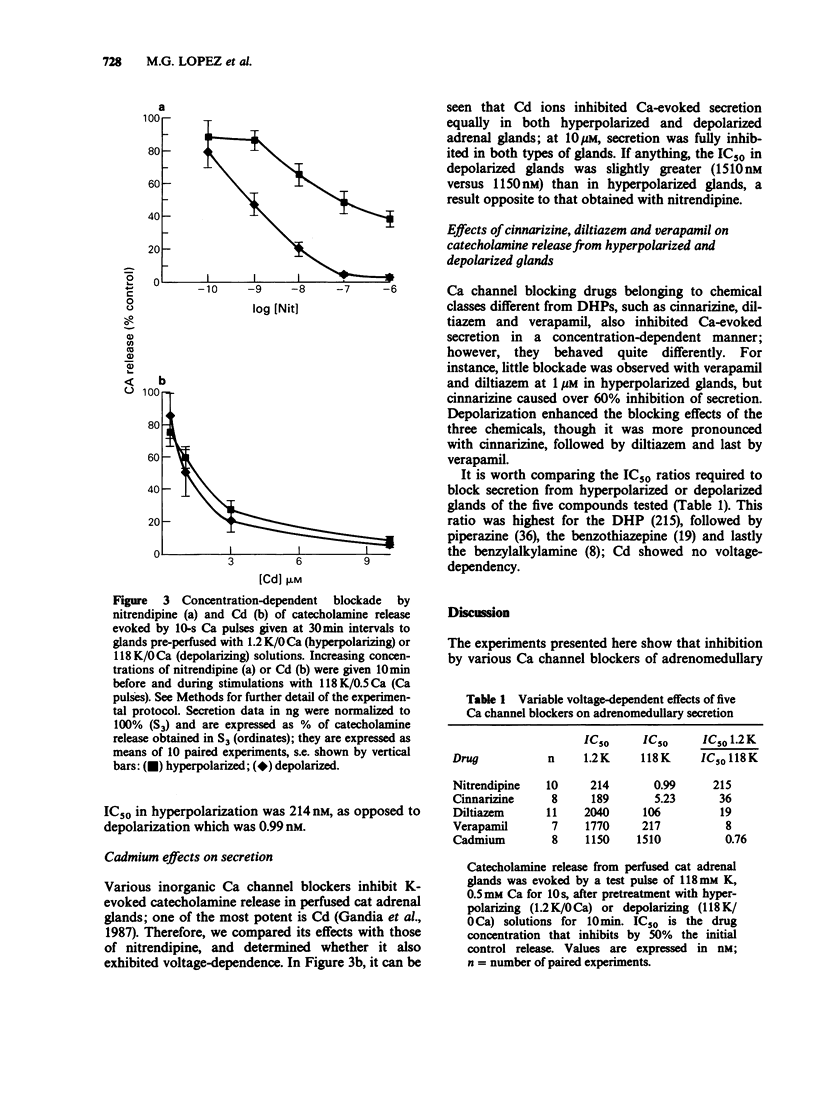
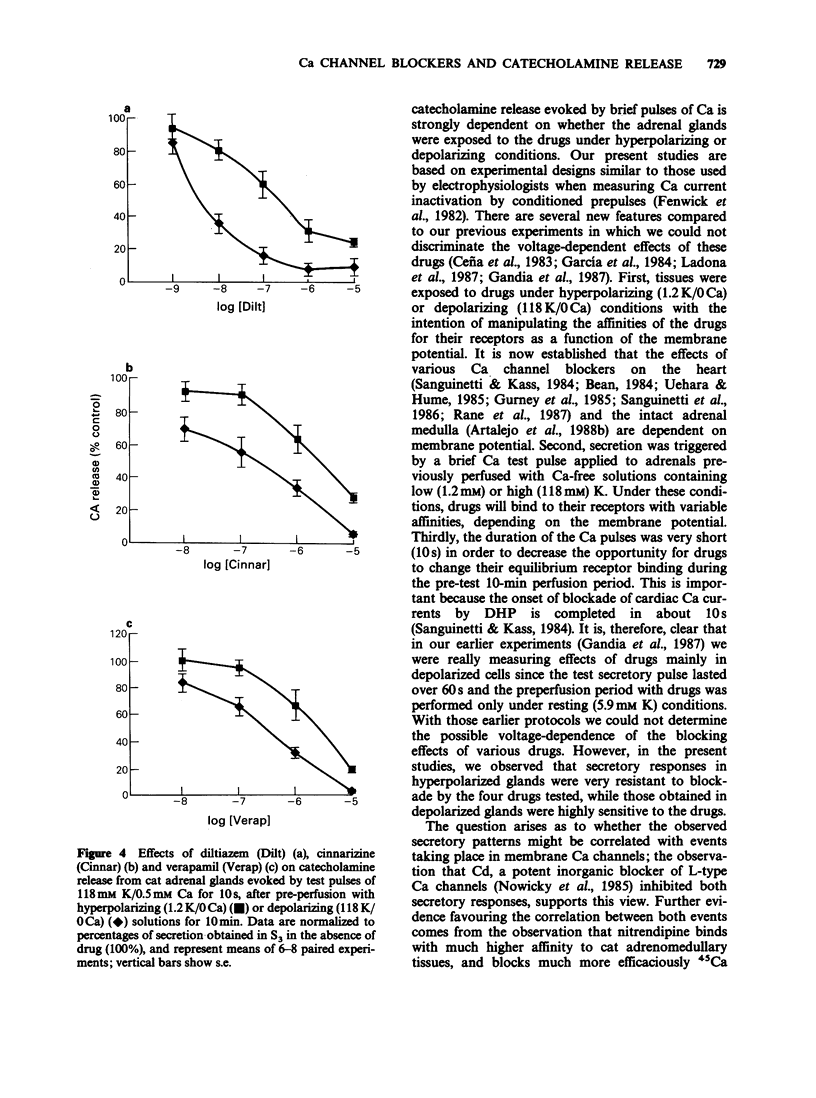
1. Catecholamine release from cat adrenal glands perfused at a high rate (4 ml min-1) at 37 degrees C with modified Krebs solutions lacking Ca and containing 1.2 mM K (hyperpolarizing solution) or 118 mM K (depolarizing solution) was triggered by 10-s pulses of Ca (0.5 mM) in the presence of 118 mM K. Hyperpolarized glands released 1280 +/- 135 ng per pulse and depolarized glands 831 +/- 98 ng per pulse (n = 29). 2. While the dihydropyridine Ca channel blocker nitrendipine inhibited secretion in hyperpolarized glands with an IC50 of 214 nM, in depolarizing conditions the drug was much more potent (IC50 = 0.99 nM). In contrast, the inorganic Ca channel blocker cadmium inhibited secretion with the same potency both in hyperpolarized or depolarized glands. 3. Cinnarizine, diltiazem and verapamil exhibited intermediate degrees of voltage-dependence in blocking secretion. The IC50 ratios between hyperpolarized and depolarized glands were 215, 36, 19, 8 and 0.76 respectively for nitrendipine, cinnarizine, diltiazem, verapamil and cadmium. Because the experimental design (strong depolarization in the absence of Ca) favours the highest opening probability of Ca channels, it seems that these drugs bind preferentially to their receptors when these channels are in their open state. 4. Variable voltage-dependent effects of the five Ca channel blockers on adrenomedullary catecholamine release suggests different sites and mechanisms of action on, or near L-type Ca channels in chromaffin cells. In addition, these findings might help to explain why these drugs exhibit tissue selectivity and why they act differently in normal polarized as compared to ischaemic depolarized cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artalejo C. R., Bader M. F., Aunis D., García A. G. Inactivation of the early calcium uptake and noradrenaline release evoked by potassium in cultured chromaffin cells. Biochem Biophys Res Commun. 1986 Jan 14;134(1):1–7. doi: 10.1016/0006-291x(86)90518-8. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., García A. G., Aunis D. Chromaffin cell calcium channel kinetics measured isotopically through fast calcium, strontium, and barium fluxes. J Biol Chem. 1987 Jan 15;262(2):915–926. [PubMed] [Google Scholar]
- Artalejo C. R., López M. G., Moro M. A., Castillo C. F., de Pascual R., García A. G. Voltage-dependence of nitrendipine provides direct evidence for dihydropyridine receptor coupling to calcium channels in intact cat adrenals. Biochem Biophys Res Commun. 1988 Jun 30;153(3):912–918. doi: 10.1016/s0006-291x(88)81314-7. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceña V., Nicolas G. P., Sanchez-Garcia P., Kirpekar S. M., Garcia A. G. Pharmacological dissection of receptor-associated and voltage-sensitive ionic channels involved in catecholamine release. Neuroscience. 1983 Dec;10(4):1455–1462. doi: 10.1016/0306-4522(83)90126-4. [DOI] [PubMed] [Google Scholar]
- Cohen C. J., McCarthy R. T. Nimodipine block of calcium channels in rat anterior pituitary cells. J Physiol. 1987 Jun;387:195–225. doi: 10.1113/jphysiol.1987.sp016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePover A., Grupp I. L., Grupp G., Schwartz A. Diltiazem potentiates the negative inotropic action of nimodipine in heart. Biochem Biophys Res Commun. 1983 Aug 12;114(3):922–929. doi: 10.1016/0006-291x(83)90648-4. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteriz R. I., Gandia L., Lopez M. G., Artalejo C. R., García A. G. Dihydropyridine chirality at the chromaffin cell calcium channel. Brain Res. 1987 Apr 7;408(1-2):359–362. doi: 10.1016/0006-8993(87)90405-7. [DOI] [PubMed] [Google Scholar]
- Gandía L., López M. G., Fonteríz R. I., Artalejo C. R., García A. G. Relative sensitivities of chromaffin cell calcium channels to organic and inorganic calcium antagonists. Neurosci Lett. 1987 Jun 26;77(3):333–338. doi: 10.1016/0304-3940(87)90523-4. [DOI] [PubMed] [Google Scholar]
- García A. G., Sala F., Reig J. A., Viniegra S., Frías J., Fontériz R., Gandía L. Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature. 1984 May 3;309(5963):69–71. doi: 10.1038/309069a0. [DOI] [PubMed] [Google Scholar]
- Gurney A. M., Nerbonne J. M., Lester H. A. Photoinduced removal of nifedipine reveals mechanisms of calcium antagonist action on single heart cells. J Gen Physiol. 1985 Sep;86(3):353–379. doi: 10.1085/jgp.86.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Ladona M. G., Aunis D., Gandía L., García A. G. Dihydropyridine modulation of the chromaffin cell secretory response. J Neurochem. 1987 Feb;48(2):483–490. doi: 10.1111/j.1471-4159.1987.tb04118.x. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Montiel C., Artalejo A. R., García A. G. Effects of the novel dihydropyridine BAY-K-8644 on adrenomedullary catecholamine release evoked by calcium reintroduction. Biochem Biophys Res Commun. 1984 May 16;120(3):851–857. doi: 10.1016/s0006-291x(84)80185-0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Rane S. G., Holz G. G., 4th, Dunlap K. Dihydropyridine inhibition of neuronal calcium current and substance P release. Pflugers Arch. 1987 Aug;409(4-5):361–366. doi: 10.1007/BF00583789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala F., Fonteriz R. I., Borges R., García A. G. Inactivation of potassium-evoked adrenomedullary catecholamine release in the presence of calcium, strontium or BAY-K-8644. FEBS Lett. 1986 Feb 3;196(1):34–38. doi: 10.1016/0014-5793(86)80209-5. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984 Sep;55(3):336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Krafte D. S., Kass R. S. Voltage-dependent modulation of Ca channel current in heart cells by Bay K8644. J Gen Physiol. 1986 Sep;88(3):369–392. doi: 10.1085/jgp.88.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellenberger M. K., Gordon J. H. A rapid, simplified procedure for simultaneous assay of norepinephrine, dopamine, and 5-hydroxytryptamine from discrete brain areas. Anal Biochem. 1971 Feb;39(2):356–372. doi: 10.1016/0003-2697(71)90426-x. [DOI] [PubMed] [Google Scholar]
- Spedding M. Functional interactions of calcium-antagonists in K+-depolarized smooth muscle. Br J Pharmacol. 1983 Nov;80(3):485–488. doi: 10.1111/j.1476-5381.1983.tb10719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggle D. J., Janis R. A. Calcium channel ligands. Annu Rev Pharmacol Toxicol. 1987;27:347–369. doi: 10.1146/annurev.pa.27.040187.002023. [DOI] [PubMed] [Google Scholar]
- Uehara A., Hume J. R. Interactions of organic calcium channel antagonists with calcium channels in single frog atrial cells. J Gen Physiol. 1985 May;85(5):621–647. doi: 10.1085/jgp.85.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif F., Triggle D. J. Functional interactions between organic calcium channel antagonists in smooth muscle. Can J Physiol Pharmacol. 1985 Mar;63(3):193–195. doi: 10.1139/y85-036. [DOI] [PubMed] [Google Scholar]


