Abstract
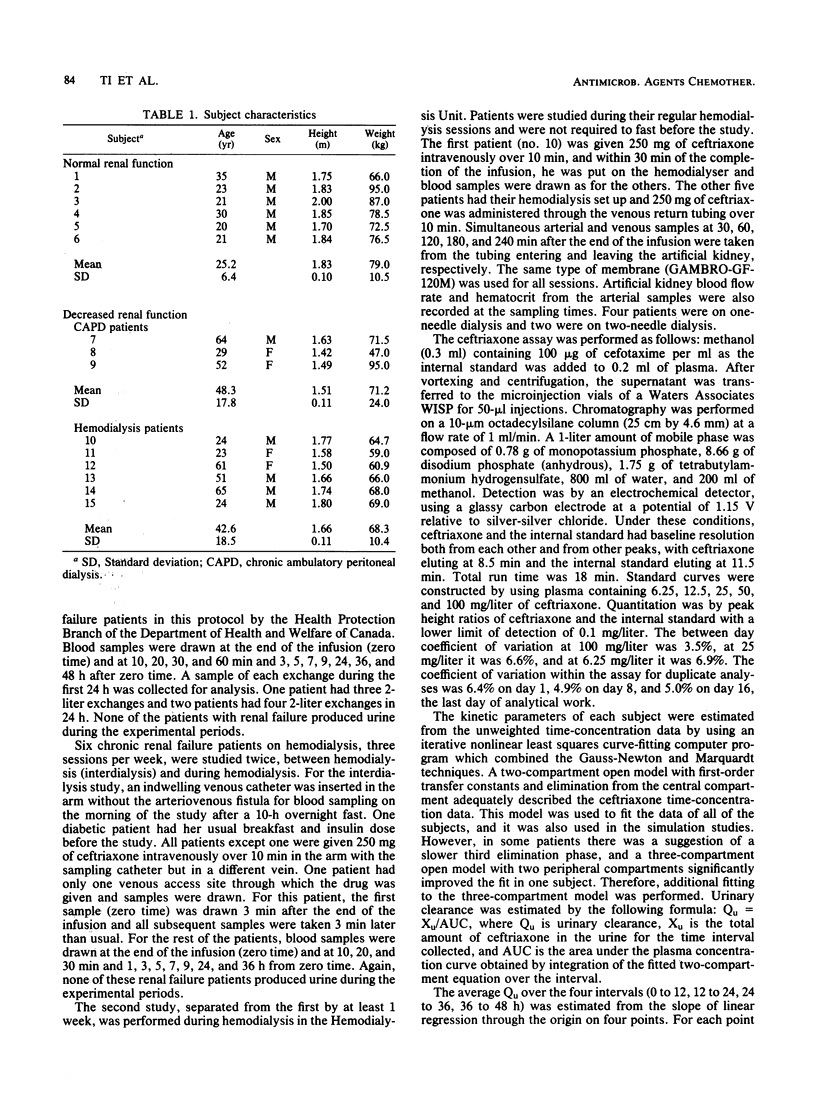
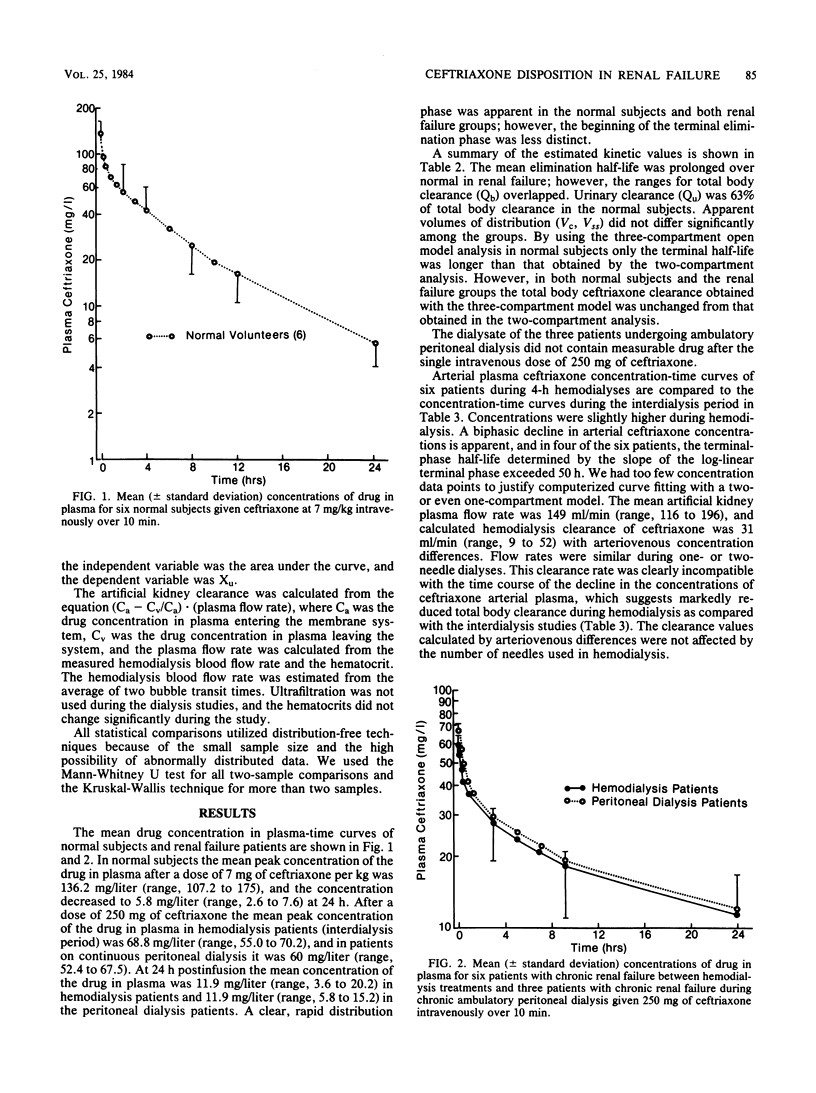
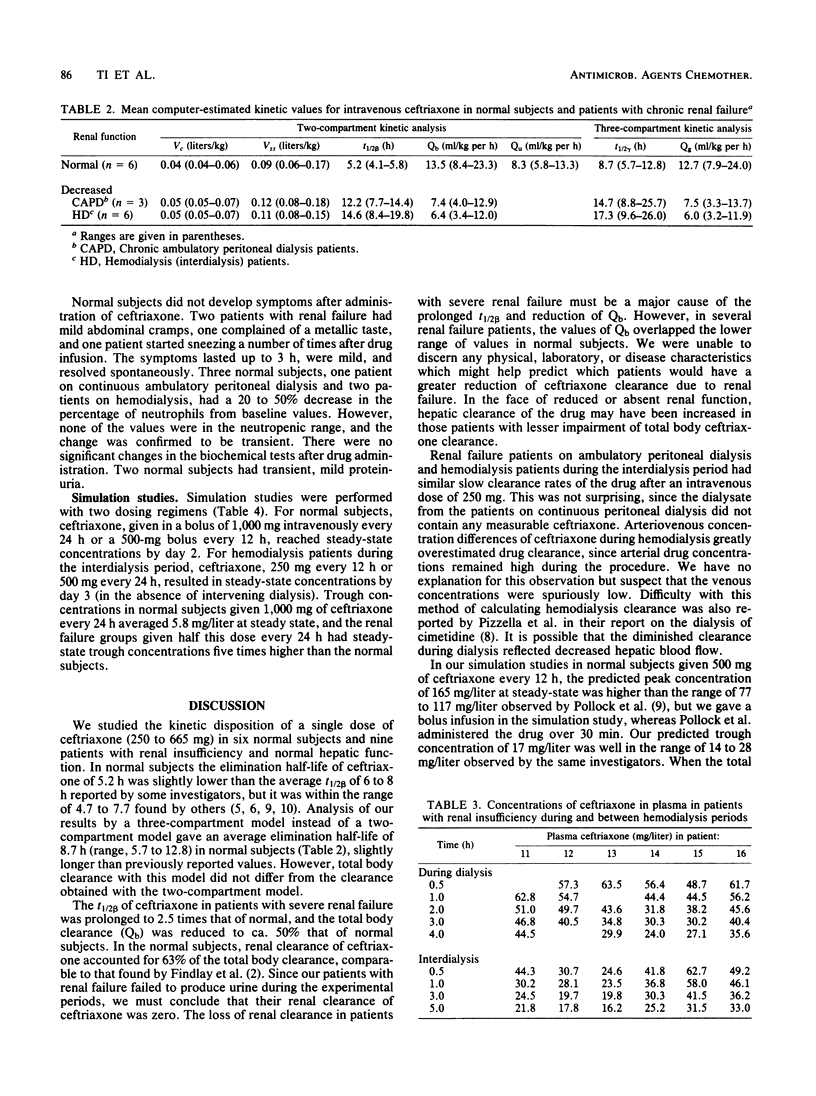
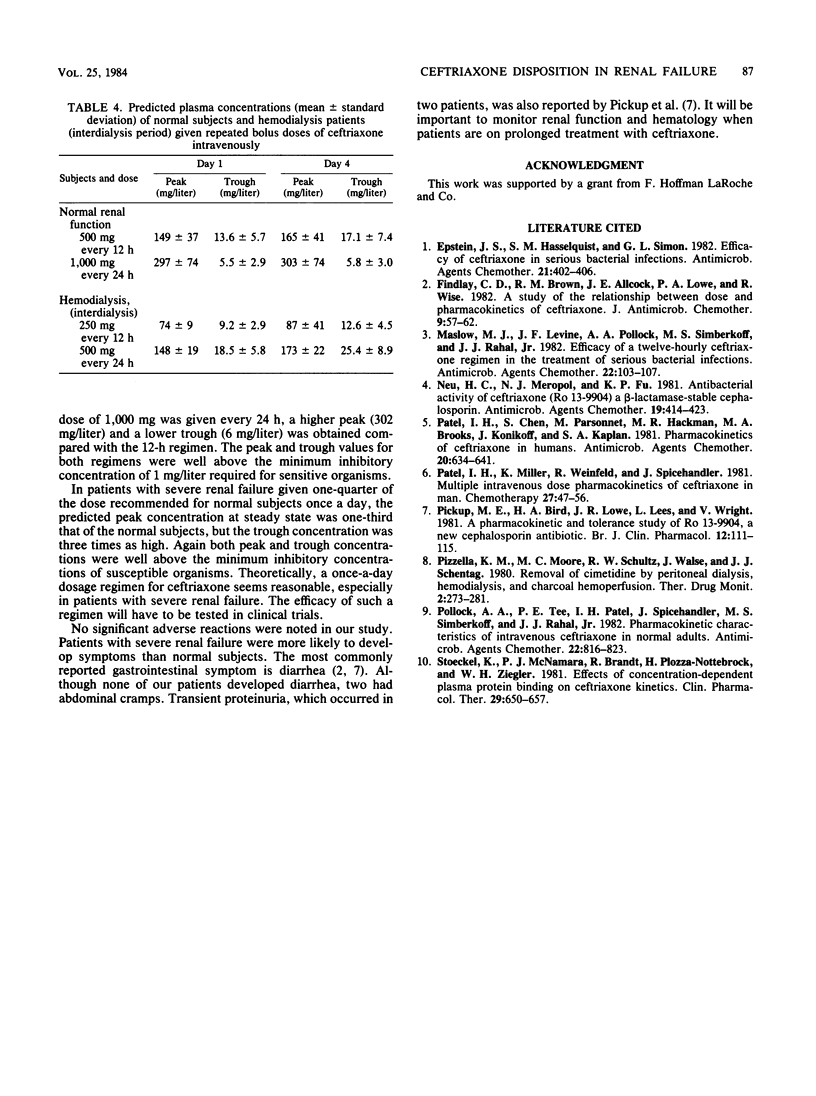
The kinetic disposition of a single intravenous dose of ceftriaxone (250 to 665 mg) was studied in six normal subjects and nine patients with renal insufficiency and normal hepatic function. In normal subjects, ceftriaxone was eliminated with a t1/2 beta of 5.2 h (range, 4.1 to 5.8). The total body clearance (Qb) was 13.5 ml/kg per h (range, 8.4 to 23.3), and renal clearance was 8.3 ml/kg per h (range, 5.8 to 13.3). In patients with severe renal insufficiency requiring peritoneal or hemodialysis, the mean t1/2 beta was prolonged to 13.4 h (range, 7.7 to 15.8) and the mean Qb was reduced to 6.9 ml/kg per h (range, 3.4 to 12.8). The apparent volumes of distribution (Vc and Vss) were not different from those determined in normal subjects. Peritoneal dialysis did not remove ceftriaxone. The dialysate of three patients on continuous peritoneal dialysis did not contain any measureable ceftriaxone, and the kinetic disposition in these patients was similar to the hemodialysis patients between their dialysis treatment. During a 4-h hemodialysis session, the total body clearance of ceftriaxone was reduced, perhaps secondary to a decrease in hepatic blood flow induced by the hemodialysis procedure. After a 12- or 24-h dose regimen, predicted trough concentrations of ceftriaxone in plasma at steady state derived from kinetic data generated from the study and assuming linear pharmacokinetic behavior were well above the minimum inhibitory concentrations of most sensitive bacteria, suggesting the feasibility of a once-a-day dosage regimen especially for patients with severe renal insufficiency.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Epstein J. S., Hasselquist S. M., Simon G. L. Efficacy of ceftriaxone in serious bacterial infections. Antimicrob Agents Chemother. 1982 Mar;21(3):402–406. doi: 10.1128/aac.21.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay C. D., Brown R. M., Allcock J. E., Lowe P. A., Wise R. A study of the relationship between dose and pharmacokinetics of ceftriaxone. J Antimicrob Chemother. 1982 Jan;9(1):57–62. doi: 10.1093/jac/9.1.57. [DOI] [PubMed] [Google Scholar]
- Maslow M. J., Levine J. F., Pollock A. A., Simberkoff M. S., Rahal J. J., Jr Efficacy of a twelve-hourly ceftriaxone regimen in the treatment of serious bacterial infections. Antimicrob Agents Chemother. 1982 Jul;22(1):103–107. doi: 10.1128/aac.22.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Meropol N. J., Fu K. P. Antibacterial activity of ceftriaxone (Ro 13-9904), a beta-lactamase-stable cephalosporin. Antimicrob Agents Chemother. 1981 Mar;19(3):414–423. doi: 10.1128/aac.19.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel I. H., Chen S., Parsonnet M., Hackman M. R., Brooks M. A., Konikoff J., Kaplan S. A. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother. 1981 Nov;20(5):634–641. doi: 10.1128/aac.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel I. H., Miller K., Weinfeld R., Spicehandler J. Multiple intravenous dose pharmacokinetics of ceftriaxone in man. Chemotherapy. 1981;27 (Suppl 1):47–56. doi: 10.1159/000238029. [DOI] [PubMed] [Google Scholar]
- Pickup M. E., Bird H. A., Lowe J. R., Lees L., Wright V. A pharmacokinetic and tolerance study of Ro13-9904, a new cephalosporin antibiotic. Br J Clin Pharmacol. 1981 Aug;12(2):111–115. doi: 10.1111/j.1365-2125.1981.tb01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzella K. M., Moore M. C., Schultz R. W., Walshe J., Schentag J. J. Removal of cimetidine by peritoneal dialysis, hemodialysis, and charcoal hemoperfusion. Ther Drug Monit. 1980;2(3):273–281. doi: 10.1097/00007691-198007000-00011. [DOI] [PubMed] [Google Scholar]
- Pollock A. A., Tee P. E., Patel I. H., Spicehandler J., Simberkoff M. S., Rahal J. J., Jr Pharmacokinetic characteristics of intravenous ceftriaxone in normal adults. Antimicrob Agents Chemother. 1982 Nov;22(5):816–823. doi: 10.1128/aac.22.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel K., McNamara P. J., Brandt R., Plozza-Nottebrock H., Ziegler W. H. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin Pharmacol Ther. 1981 May;29(5):650–657. doi: 10.1038/clpt.1981.90. [DOI] [PubMed] [Google Scholar]


