Abstract
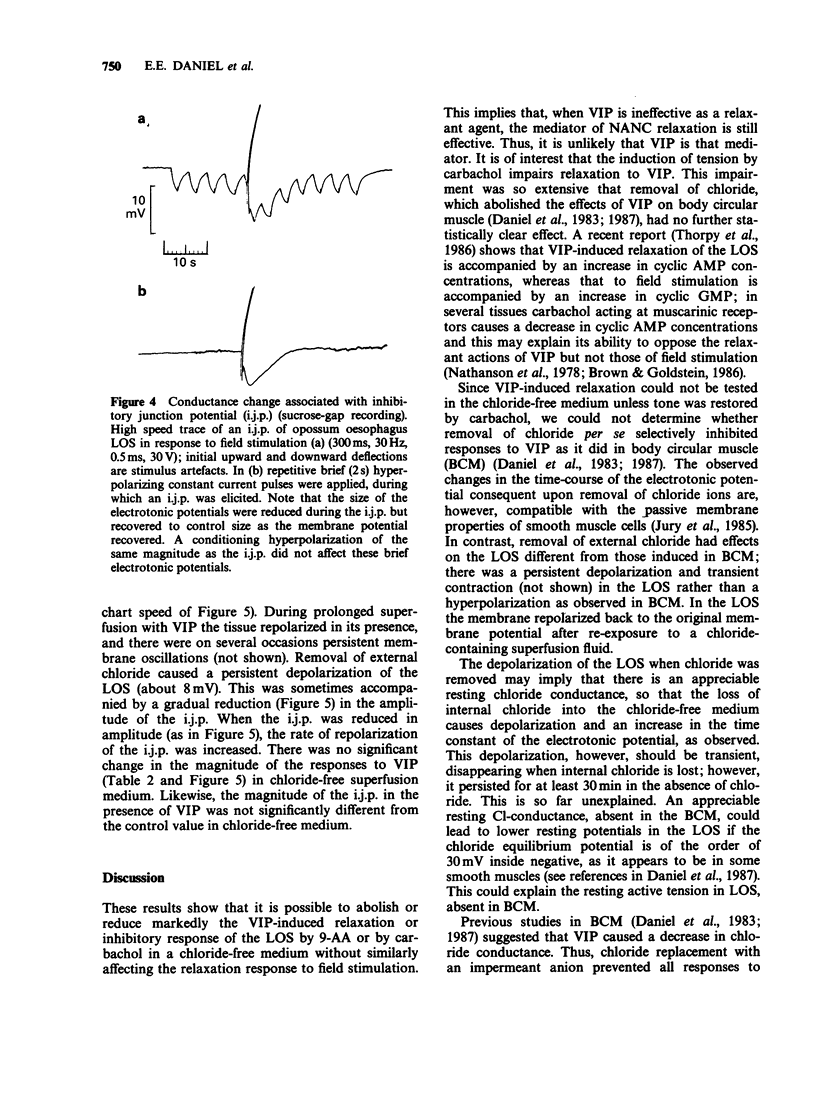
1. Field stimulation or vasoactive intestinal polypeptide (VIP) relaxed lower oesophageal sphincter (LOS) from North American opossum. Pretreatment with carbachol in Cl-ion-containing or Cl-ion-free Krebs solution or with 10(-3) M 9-aminoacridine abolished or markedly reduced relaxation due to VIP applied exogenously but not that elicited by field stimulation of non-adrenergic, non-cholinergic nerves. 2. Inhibitory junction potentials (7.5 +/- 1.2 mV, n = 5) could be recorded in LOS strips with the sucrose gap technique. They lacked significant after-depolarizations but were accompanied by decreased membrane resistance (61 +/- 6%, n = 3). In these strips, VIP (10(-6) M) produced small hyperpolarizations (2.1 +/- 1.1 mV, n = 5) sometimes followed by membrane potential oscillations but no change in conductance. 3. Removal of external chloride depolarized the membranes (7.6 +/- 1.7 mV) but did not prevent the hyperpolarization to VIP or the occurrence of inhibitory junction potentials. Restoration of external chloride repolarized the cells. It appears that an appreciable chloride conductance may be present in sphincter muscle cells and this may cause them to be more depolarized than non-sphincter muscle. 4. We conclude that it is very unlikely that VIP is the inhibitory NANC neurotransmitter since it does not mimic the inhibitory junction potential.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. H., Goldstein D. Differences in muscarinic receptor reserve for inhibition of adenylate cyclase and stimulation of phosphoinositide hydrolysis in chick heart cells. Mol Pharmacol. 1986 Dec;30(6):566–570. [PubMed] [Google Scholar]
- Bywater R. A., Holman M. E., Taylor G. S. Atropine-resistant depolarization in the guinea-pig small intestine. J Physiol. 1981 Jul;316:369–378. doi: 10.1113/jphysiol.1981.sp013794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E. E., Crankshaw J., Sarna S. Prostaglandins and tetrodotoxin-insensitive relaxation of opossum lower esophageal sphincter. Am J Physiol. 1979 Feb;236(2):E153–E172. doi: 10.1152/ajpendo.1979.236.2.E153. [DOI] [PubMed] [Google Scholar]
- Daniel E. E., Helmy-Elkholy A., Jager L. P., Kannan M. S. Neither a purine nor VIP is the mediator of inhibitory nerves of opossum oesophageal smooth muscle. J Physiol. 1983 Mar;336:243–260. doi: 10.1113/jphysiol.1983.sp014579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E. E., Jager L. P., Jury J. Catecholamines release mediators in the opossum oesophageal circular smooth muscle. J Physiol. 1987 Jan;382:489–508. doi: 10.1113/jphysiol.1987.sp016380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E. E., Posey-Daniel V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol. 1984 Mar;246(3 Pt 1):G305–G315. doi: 10.1152/ajpgi.1984.246.3.G305. [DOI] [PubMed] [Google Scholar]
- Daniel E. E., Sarna S., Waterfall W., Crankshaw J. Role of endogenous prostaglandins in regulating the tone of opossum lower esophageal sphincter in vivo. Prostaglandins. 1979 Apr;17(4):641–648. doi: 10.1016/0090-6980(79)90015-7. [DOI] [PubMed] [Google Scholar]
- Goyal R. K., Rattan S. Neurohumoral, hormonal, and drug receptors for the lower esophageal sphincter. Gastroenterology. 1978 Mar;74(3):598–619. [PubMed] [Google Scholar]
- Goyal R. K., Rattan S., Said S. I. VIP as a possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurones. Nature. 1980 Nov 27;288(5789):378–380. doi: 10.1038/288378a0. [DOI] [PubMed] [Google Scholar]
- Jury J., Jager L. P., Daniel E. E. Unusual potassium channels mediate nonadrenergic noncholinergic nerve-mediated inhibition in opossum esophagus. Can J Physiol Pharmacol. 1985 Feb;63(2):107–112. doi: 10.1139/y85-020. [DOI] [PubMed] [Google Scholar]
- Nathanson N. M., Klein W. L., Nirenberg M. Regulation of adenylate cyclase activity mediated by muscarinic acetylcholine receptors. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1788–1791. doi: 10.1073/pnas.75.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S., Goyal R. K. Neural control of the lower esophageal sphincter: influence of the vagus nerves. J Clin Invest. 1974 Oct;54(4):899–906. doi: 10.1172/JCI107829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S., Grady M., Goyal R. K. Vasoactive intestinal peptide causes peristaltic contractions in the esophageal body. Life Sci. 1982 May 3;30(18):1557–1563. doi: 10.1016/0024-3205(82)90244-2. [DOI] [PubMed] [Google Scholar]
- Sarna S. K., Daniel E. E., Waterfall W. E. Myogenic and neural control systems for esophageal motility. Gastroenterology. 1977 Dec;73(6):1345–1352. [PubMed] [Google Scholar]
- Tomita T. Conductance change during the inhibitory potential in the guinea-pig taenia coli. J Physiol. 1972 Sep;225(3):693–703. doi: 10.1113/jphysiol.1972.sp009964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torphy T. J., Fine C. F., Burman M., Barnette M. S., Ormsbee H. S., 3rd Lower esophageal sphincter relaxation is associated with increased cyclic nucleotide content. Am J Physiol. 1986 Dec;251(6 Pt 1):G786–G793. doi: 10.1152/ajpgi.1986.251.6.G786. [DOI] [PubMed] [Google Scholar]
- Volle R. L. Blockade by 9-aminoacridine of potassium fluxes in frog sartorius muscle. Biochem Pharmacol. 1971 Feb;20(2):315–324. doi: 10.1016/0006-2952(71)90066-9. [DOI] [PubMed] [Google Scholar]


