Abstract
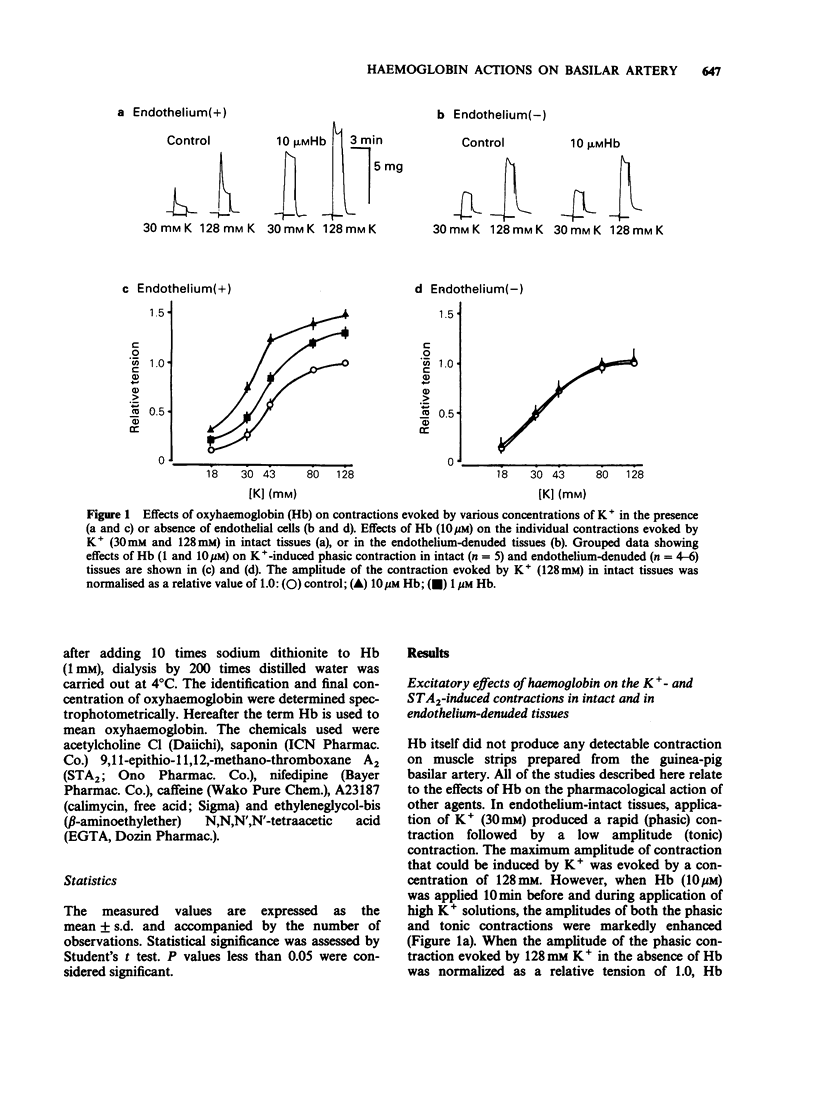
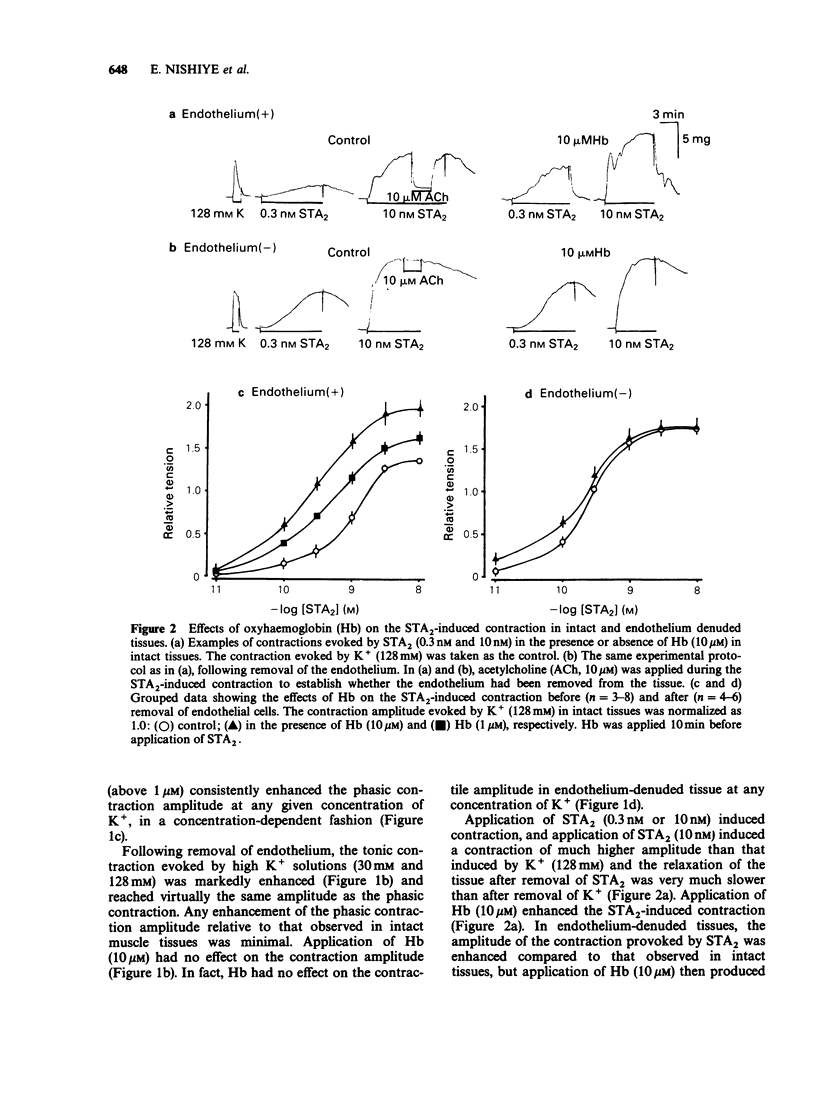
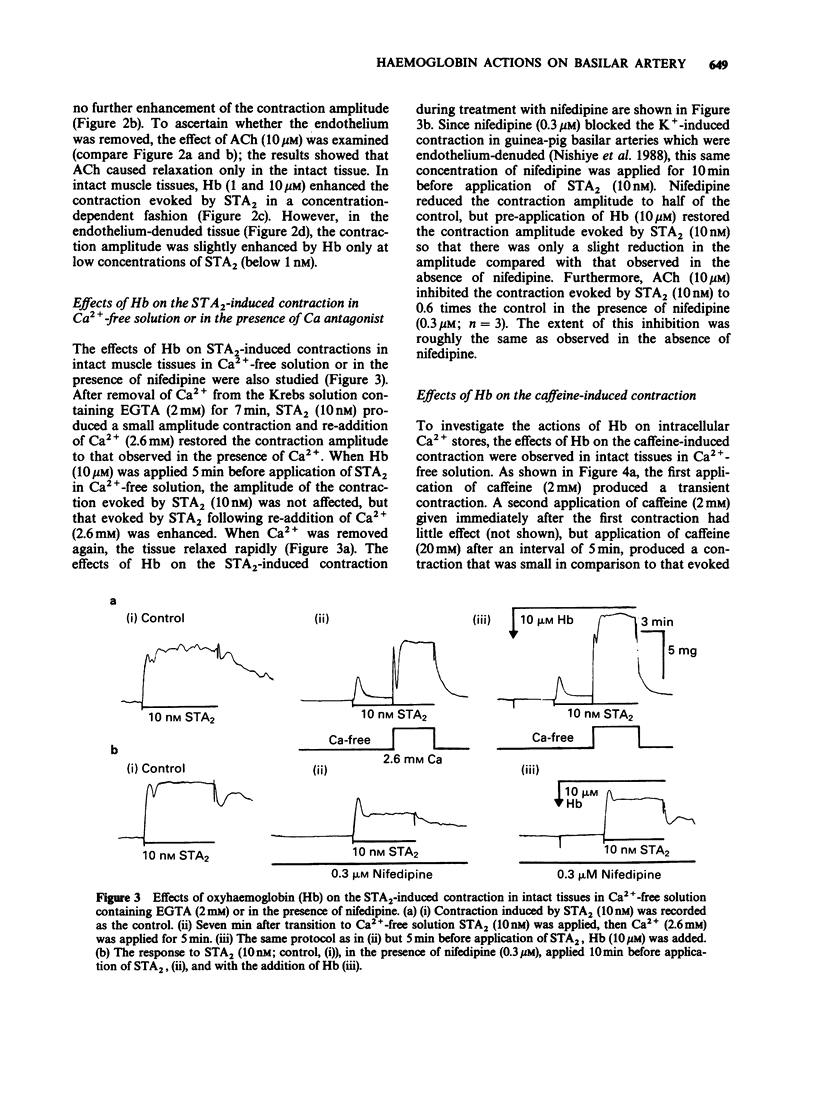
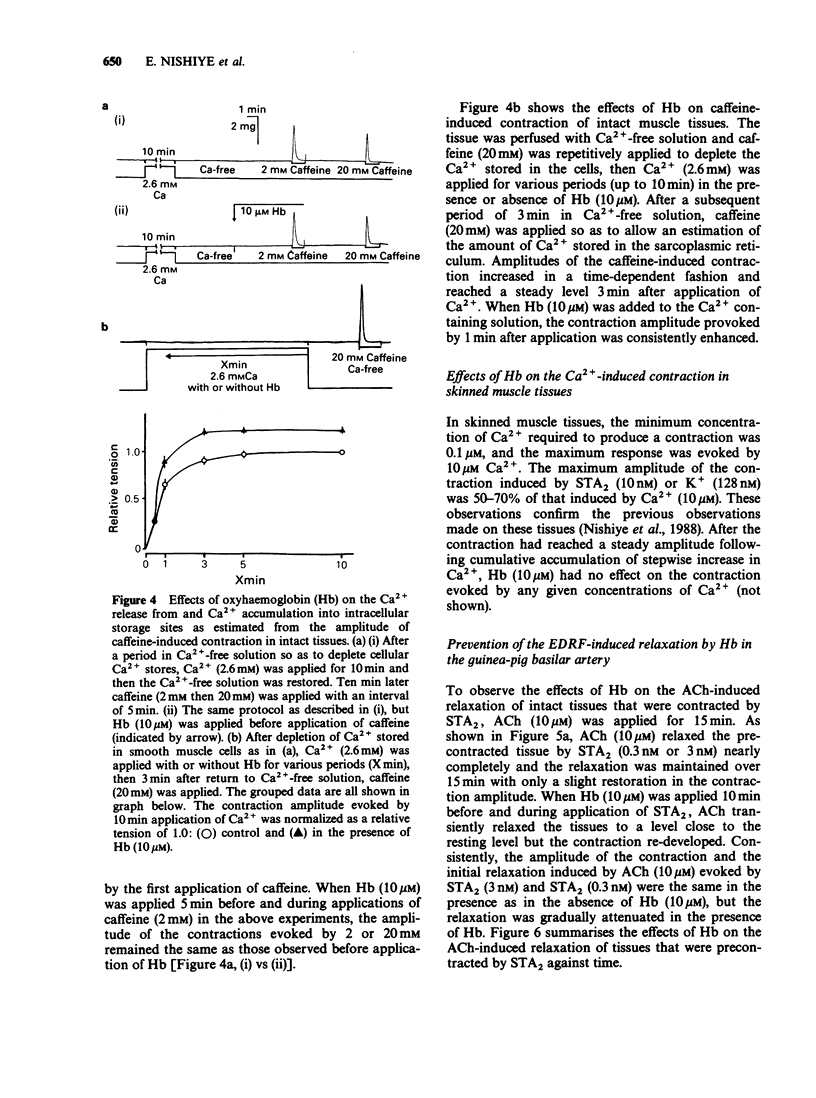
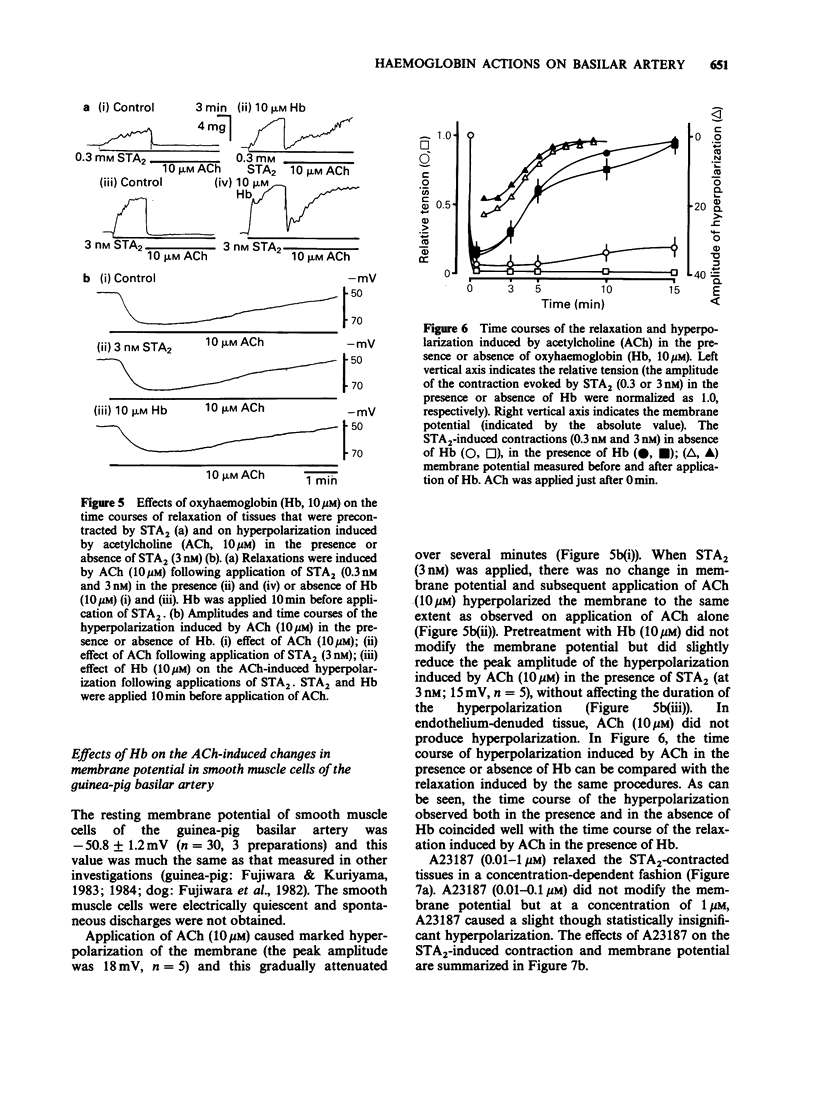
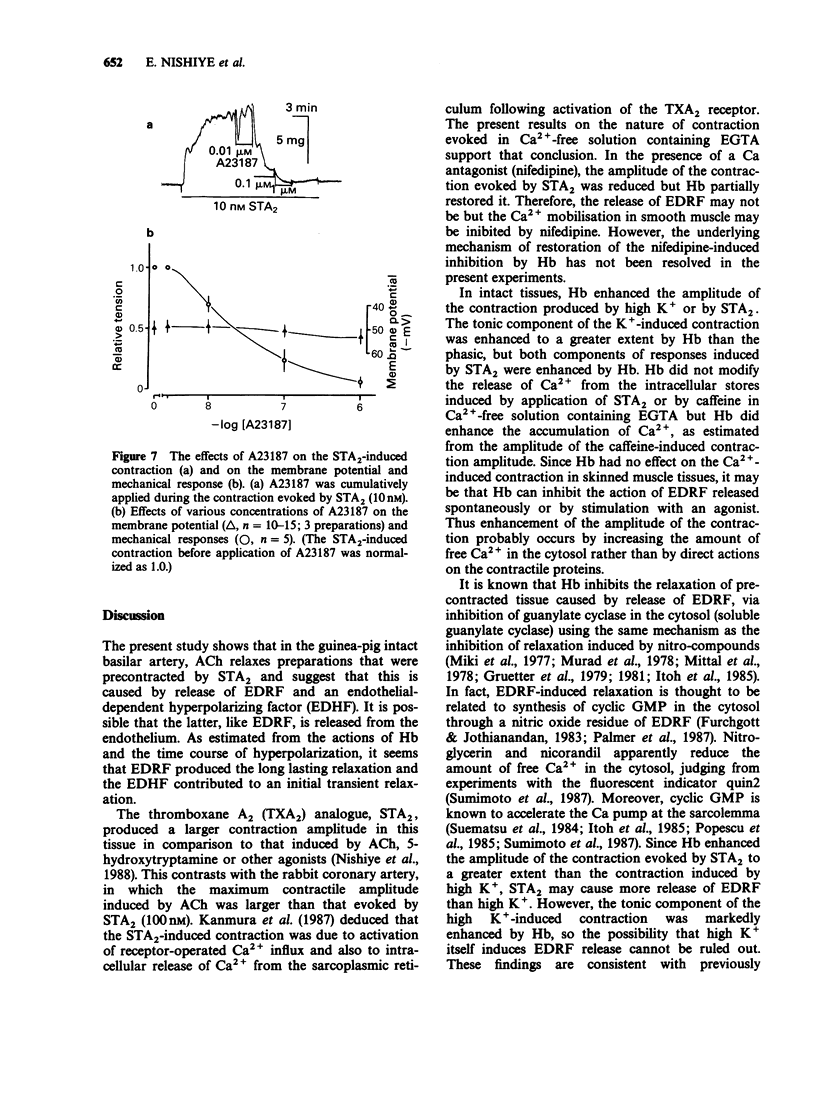
1. Factors inducing dilatation of guinea-pig basilar artery were investigated in intact and endothelium-denuded tissues by measurement of isometric tension and by electrophysiological methods. 2. The amplitudes of contractions induced by 9,11,epithio-11,12-methanothromboxane A2 (STA2) and by high K+ were enhanced by haemoglobin (oxyhaemoglobin, Hb) in a concentration-dependent fashion (above 1 microM). For the high K+-induced contraction, the initial tonic component was enhanced to a greater extent than the secondary phasic component. Mechanical responses evoked by STA2 and by high K+ were greater in endothelium-denuded tissues, but Hb (below 10 microM) had no effect on them. 3. Hb (10 microM) had no effect on the contractile proteins as estimated from the actions of Hb on Ca2+-induced contractions in skinned muscle tissues. Further, Hb had no effect on the release of Ca2+ from intracellular stores but it accelerated the Ca2+ accumulation into the sarcoplasmic reticulum as judged from the caffeine- or STA2-induced contraction generated in intact tissues. 4. Acetylcholine (ACh) relaxed tissues that were precontracted by STA2 but Hb prevented this relaxation, in a concentration-dependent fashion. The ACh-induced relaxation was sustained for over 10 min in the absence of Hb, but following application of Hb, ACh caused only a transient relaxation. 5. STA2 (up to 100 nM) did not modify the resting membrane potential of smooth muscle cells of the basilar artery. ACh (10 microM) caused transient hyperpolarization which was only slightly inhibited by Hb (10 microM) whether or not STA2 was present. The hyperpolarization induced by ACh required the presence of endothelial cells. 6. A23187 (0.01-1 microM) relaxed tissues which were precontracted by STA2, in a concentration-dependent fashion but had no effect on the membrane potential. 7. These results suggest that in guinea-pig basilar artery, ACh induces relaxation of tissues that were precontracted by STA2 by causing release of both endothelium-derived relaxing (EDRF) and endothelial dependent hyperpolarizing factor (EDHF) (sustained and initial transient relaxation, respectively), but via different mechanisms. Hb inhibits the former and to a lesser extent, the latter. Since A23187 produced relaxation of pre-contracted tissue but caused no detectable change in the membrane potential, this agent may release EDRF but not EDHF.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger N. L., Dillender M. J., Majerus P. W. Cultured human skin fibroblasts and arterial cells produce a labile platelet-inhibitory prostaglandin. Biochem Biophys Res Commun. 1977 Sep 9;78(1):294–301. doi: 10.1016/0006-291x(77)91253-0. [DOI] [PubMed] [Google Scholar]
- Beny J. L., Brunet P. C., Huggel H. Effect of mechanical stimulation, substance P and vasoactive intestinal polypeptide on the electrical and mechanical activities of circular smooth muscles from pig coronary arteries contracted with acetylcholine: role of endothelium. Pharmacology. 1986;33(2):61–68. doi: 10.1159/000138202. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Clapp L. H. Endothelial-dependent relaxant actions of carbachol and substance P in arterial smooth muscle. Br J Pharmacol. 1986 Apr;87(4):713–723. doi: 10.1111/j.1476-5381.1986.tb14589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busija D. W., Leffler C. W., Beasley D. G. Effects of leukotrienes C4, D4, and E4 on cerebral arteries of newborn pigs. Pediatr Res. 1986 Oct;20(10):973–976. doi: 10.1203/00006450-198610000-00016. [DOI] [PubMed] [Google Scholar]
- Cherry P. D., Furchgott R. F., Zawadzki J. V., Jothianandan D. Role of endothelial cells in relaxation of isolated arteries by bradykinin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2106–2110. doi: 10.1073/pnas.79.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks T. M., Angus J. A., Campbell J. H., Campbell G. R. Release and properties of endothelium-derived relaxing factor (EDRF) from endothelial cells in culture. J Cell Physiol. 1985 Jun;123(3):310–320. doi: 10.1002/jcp.1041230304. [DOI] [PubMed] [Google Scholar]
- Connor H. E., Feniuk W. Role of endothelium in haemoglobin-induced contraction of dog basilar artery. Eur J Pharmacol. 1987 Aug 4;140(1):105–108. doi: 10.1016/0014-2999(87)90640-6. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Anoxia and endothelium-dependent reactivity of the canine femoral artery. J Physiol. 1983 Feb;335:65–74. doi: 10.1113/jphysiol.1983.sp014519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J., Chu E. B. Possible role for cyclic GMP in endothelium-dependent relaxation of rabbit aorta by acetylcholine. Comparison with nitroglycerin. Res Commun Chem Pathol Pharmacol. 1983 Sep;41(3):369–381. [PubMed] [Google Scholar]
- Domae M., Kuriyama H. Effects of prostaglandin I2 synthesized in the endothelium and in the smooth muscle on mechanical properties of the canine thoracic aorta. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jul;333(3):294–302. doi: 10.1007/BF00512944. [DOI] [PubMed] [Google Scholar]
- Eldor A., Falcone D. J., Hajjar D. P., Minick C. R., Weksler B. B. Recovery of prostacyclin production by de-endothelialized rabbit aorta. Critical role of neointimal smooth muscle cells. J Clin Invest. 1981 Mar;67(3):735–741. doi: 10.1172/JCI110090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C. M., Kistler J. P., Davis J. M. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980 Jan;6(1):1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Itoh T., Suzuki H. Membrane properties and excitatory neuromuscular transmission in the smooth muscle of dog cerebral arteries. Br J Pharmacol. 1982 Oct;77(2):197–208. doi: 10.1111/j.1476-5381.1982.tb09286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S., Kassell N. F., Sasaki T., Nakagomi T., Lehman R. M. Selective hemoglobin inhibition of endothelium-dependent vasodilation of rabbit basilar artery. J Neurosurg. 1986 Mar;64(3):445–452. doi: 10.3171/jns.1986.64.3.0445. [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Kuriyama H. Effects of agents that modulate potassium permeability on smooth muscle cells of the guinea-pig basilar artery. Br J Pharmacol. 1983 May;79(1):23–35. doi: 10.1111/j.1476-5381.1983.tb10491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S., Kuriyama H. Hemolysate-induced contraction in smooth muscle cells of the guinea pig basilar artery. Stroke. 1984 May-Jun;15(3):503–510. doi: 10.1161/01.str.15.3.503. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Henderson A. H., Edwards D. H., Lewis M. J. Isolated perfused rabbit coronary artery and aortic strip preparations: the role of endothelium-derived relaxant factor. J Physiol. 1984 Jun;351:13–24. doi: 10.1113/jphysiol.1984.sp015228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter C. A., Barry B. K., McNamara D. B., Gruetter D. Y., Kadowitz P. J., Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res. 1979;5(3):211–224. [PubMed] [Google Scholar]
- Gruetter C. A., Kadowitz P. J., Ignarro L. J. Methylene blue inhibits coronary arterial relaxation and guanylate cyclase activation by nitroglycerin, sodium nitrite, and amyl nitrite. Can J Physiol Pharmacol. 1981 Feb;59(2):150–156. doi: 10.1139/y81-025. [DOI] [PubMed] [Google Scholar]
- Holzmann S. Endothelium-induced relaxation by acetylcholine associated with larger rises in cyclic GMP in coronary arterial strips. J Cyclic Nucleotide Res. 1982;8(6):409–419. [PubMed] [Google Scholar]
- Ignarro L. J., Burke T. M., Wood K. S., Wolin M. S., Kadowitz P. J. Association between cyclic GMP accumulation and acetylcholine-elicited relaxation of bovine intrapulmonary artery. J Pharmacol Exp Ther. 1984 Mar;228(3):682–690. [PubMed] [Google Scholar]
- Itoh T., Kajiwara M., Kitamura K., Kuriyama H. Roles of stored calcium on the mechanical response evoked in smooth muscle cells of the porcine coronary artery. J Physiol. 1982 Jan;322:107–125. doi: 10.1113/jphysiol.1982.sp014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmura Y., Itoh T., Kuriyama H. Mechanisms of vasoconstriction induced by 9,11-epithio-11,12-methano-thromboxane A2 in the rabbit coronary artery. Circ Res. 1987 Mar;60(3):402–409. doi: 10.1161/01.res.60.3.402. [DOI] [PubMed] [Google Scholar]
- Kassell N. F., Sasaki T., Colohan A. R., Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985 Jul-Aug;16(4):562–572. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- Komori K., Suzuki H. Electrical responses of smooth muscle cells during cholinergic vasodilation in the rabbit saphenous artery. Circ Res. 1987 Oct;61(4):586–593. doi: 10.1161/01.res.61.4.586. [DOI] [PubMed] [Google Scholar]
- Komori K., Suzuki H. Heterogeneous distribution of muscarinic receptors in the rabbit saphenous artery. Br J Pharmacol. 1987 Nov;92(3):657–664. doi: 10.1111/j.1476-5381.1987.tb11369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Miki N., Kawabe Y., Kuriyama K. Activation of cerebral guanylate cyclase by nitric oxide. Biochem Biophys Res Commun. 1977 Apr 25;75(4):851–856. doi: 10.1016/0006-291x(77)91460-7. [DOI] [PubMed] [Google Scholar]
- Mittal C. K., Arnold W. P., Murad F. Characterization of protein inhibitors of guanylate cyclase activation from rat heart and bovine lung. J Biol Chem. 1978 Feb 25;253(4):1266–1271. [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Murad F., Mittal C. K., Arnold W. P., Katsuki S., Kimura H. Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res. 1978;9:145–158. [PubMed] [Google Scholar]
- Nagao T., Suzuki H. Non-neural electrical responses of smooth muscle cells of the rabbit basilar artery to electrical field stimulation. Jpn J Physiol. 1987;37(3):497–513. doi: 10.2170/jjphysiol.37.497. [DOI] [PubMed] [Google Scholar]
- Nishiye E., Itoh T., Kuriyama H. Some effects of leukotriene D4 on the mechanical properties of the guinea-pig basilar artery. Br J Pharmacol. 1988 Mar;93(3):591–600. doi: 10.1111/j.1476-5381.1988.tb10315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Popescu L. M., Panoiu C., Hinescu M., Nutu O. The mechanism of cGMP-induced relaxation in vascular smooth muscle. Eur J Pharmacol. 1985 Jan 8;107(3):393–394. doi: 10.1016/0014-2999(85)90269-9. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rosenblum W. I. Constricting effect of leukotrienes on cerebral arterioles of mice. Stroke. 1985 Mar-Apr;16(2):262–263. doi: 10.1161/01.str.16.2.262. [DOI] [PubMed] [Google Scholar]
- Saito I., Ueda Y., Sano K. Significance of vasospasm in the treatment of ruptured intracranial aneurysms. J Neurosurg. 1977 Sep;47(3):412–429. doi: 10.3171/jns.1977.47.3.0412. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Kuriyama H. Effects of cAMP- and cGMP-dependent protein kinases, and calmodulin on Ca2+ uptake by highly purified sarcolemmal vesicles of vascular smooth muscle. Biochim Biophys Acta. 1984 Jun 13;773(1):83–90. doi: 10.1016/0005-2736(84)90552-2. [DOI] [PubMed] [Google Scholar]
- Sumimoto K., Domae M., Yamanaka K., Nakao K., Hashimoto T., Kitamura K., Kuriyama H. Actions of nicorandil on vascular smooth muscles. J Cardiovasc Pharmacol. 1987;10 (Suppl 8):S66–S75. [PubMed] [Google Scholar]
- Tagari P., Du Boulay G. H., Aitken V., Boullin D. J. Leukotriene D4 and the cerebral vasculature in vivo and in vitro. Prostaglandins Leukot Med. 1983 Jul;11(3):281–297. doi: 10.1016/0262-1746(83)90041-0. [DOI] [PubMed] [Google Scholar]
- Tanishima T. Cerebral vasospasm: contractile activity of hemoglobin in isolated canine basilar arteries. J Neurosurg. 1980 Dec;53(6):787–793. doi: 10.3171/jns.1980.53.6.0787. [DOI] [PubMed] [Google Scholar]
- Toda N., Shimizu K., Ohta T. Mechanism of cerebral arterial contraction induced by blood constituents. J Neurosurg. 1980 Sep;53(3):312–322. doi: 10.3171/jns.1980.53.3.0312. [DOI] [PubMed] [Google Scholar]
- Wellum G. R., Irvine T. W., Jr, Zervas N. T. Cerebral vasoactivity of heme proteins in vitro. Some mechanistic considerations. J Neurosurg. 1982 Jun;56(6):777–783. doi: 10.3171/jns.1982.56.6.0777. [DOI] [PubMed] [Google Scholar]
- Winquist R. J., Bunting P. B., Schofield T. L. Blockade of endothelium-dependent relaxation by the amiloride analog dichlorobenzamil: possible role of Na+/Ca++ exchange in the release of endothelium-derived relaxant factor. J Pharmacol Exp Ther. 1985 Dec;235(3):644–650. [PubMed] [Google Scholar]


