Abstract
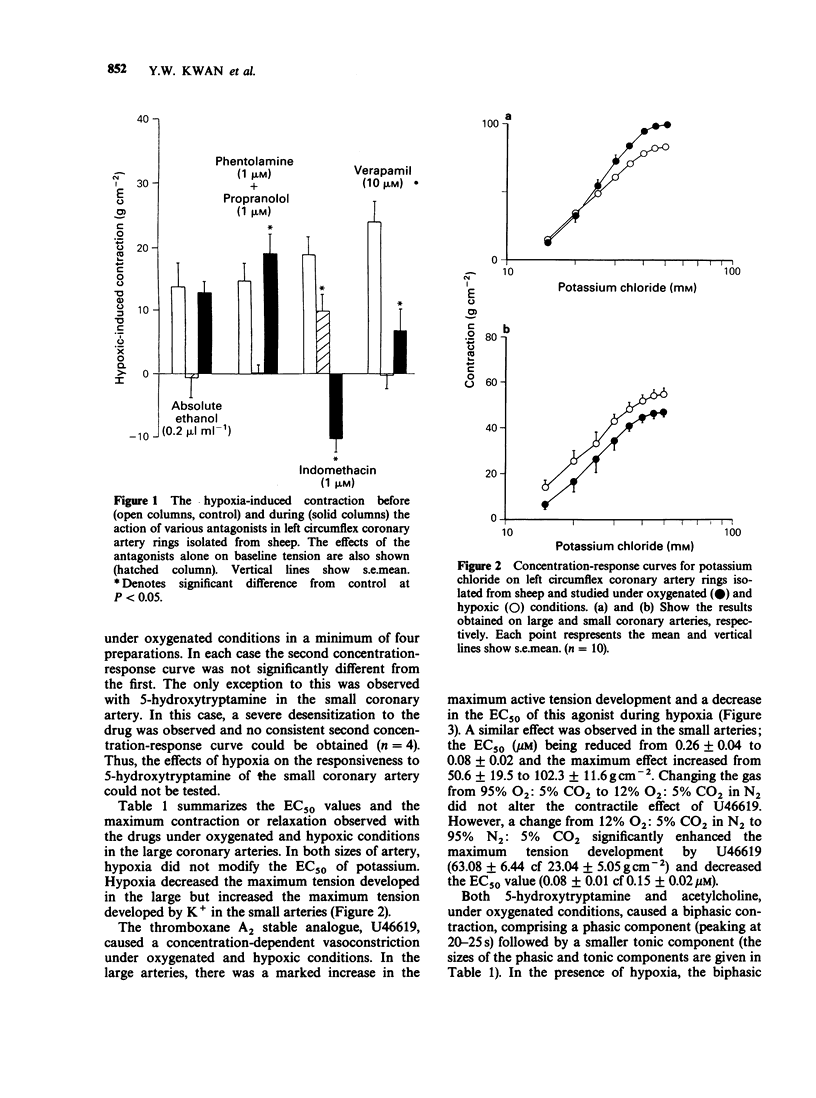
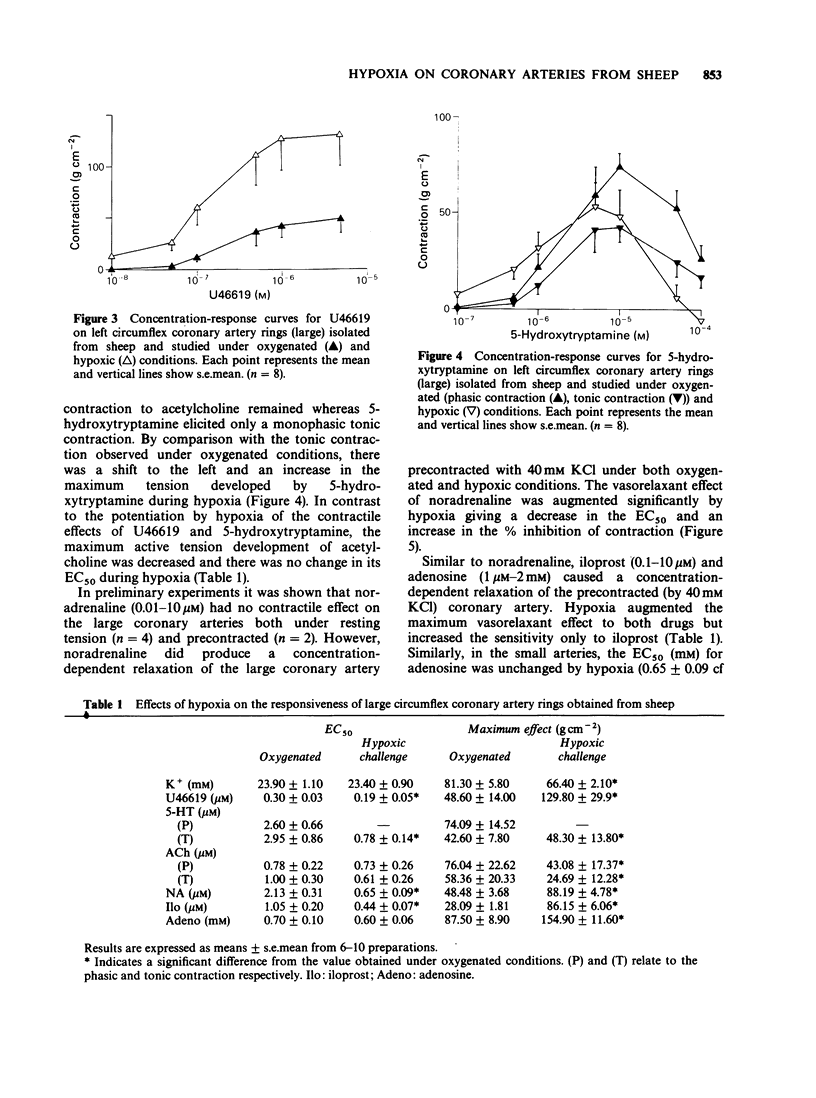
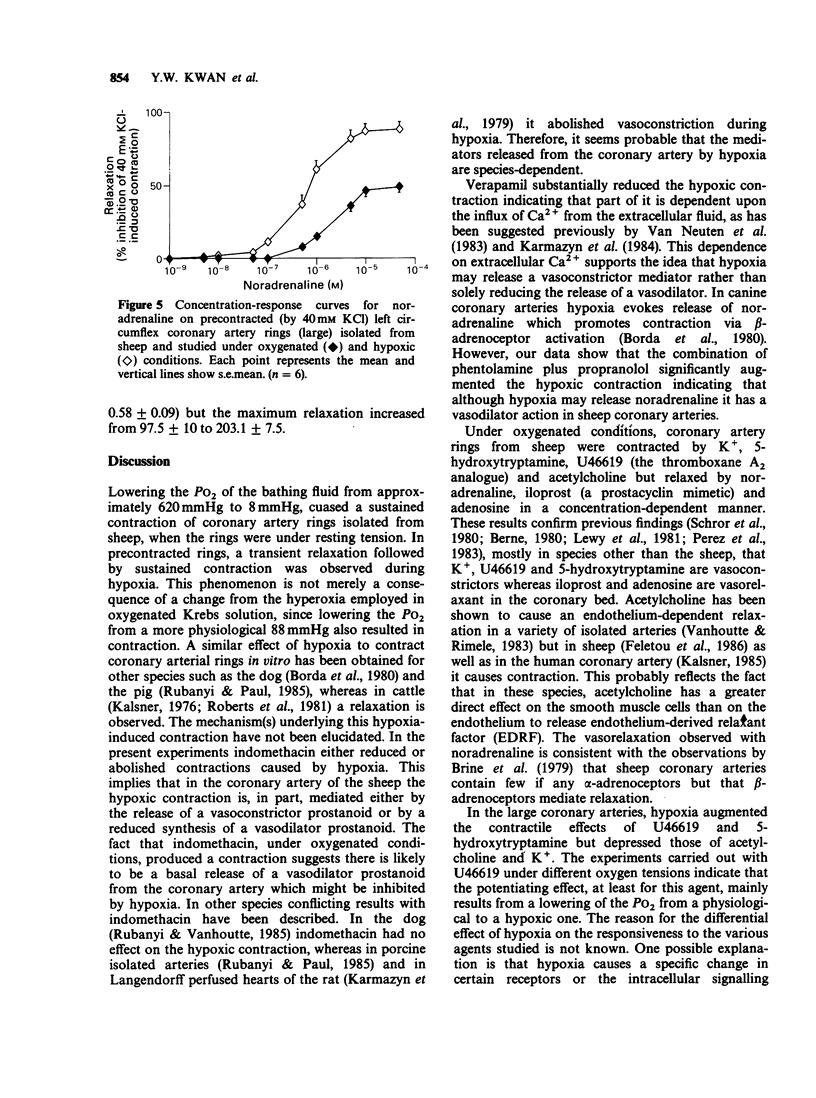
1. The effects of low oxygen tension on tone and on the responsiveness to contractile and relaxant agents were examined on circumflex coronary artery rings isolated from sheep. 2. When artery rings (2-2.5 mm o.d.) were set at their optimal resting tension, introduction of hypoxia (0% O2) caused a sustained contraction which was reversible on washing with oxygenated Krebs solution. In precontracted (40 mM KCl) arteries, hypoxia caused a similar response except that it was preceded by a transient relaxation. 3. The hypoxia-induced contraction was potentiated by the combination of phentolamine (1 microM) and propranolol (1 microM), markedly reduced by verapamil (10 microM) and either abolished or reduced by indomethacin (1 microM). Indomethacin itself caused a contraction. 4. Under hypoxic conditions, the contractile effects of U46619 (a stable thromboxane analogue) and 5-hydroxytryptamine (5-HT) and the vasodilator effects of noradrenaline, iloprost (a prostacyclin mimetic) and adenosine were markedly potentiated. In contrast, vasoconstriction to potassium or acetylcholine was depressed. 5. Changing the gases from 95% O2 to 12% O2 had no significant effect on the contractile effects of U46619. However, the maximum contractile effect of U46619 was significantly enhanced by changing the gases from 12% O2 to 0% O2. 6. Rings from a smaller branch (0.6-1.3 mm o.d.) of the circumflex coronary artery of the sheep, in the presence of hypoxia, exhibited qualitatively similar changes in the responsiveness to U46619, 5-HT and adenosine to those observed in the large artery. However, the effect of potassium was potentiated rather than depressed.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berne R. M. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980 Dec;47(6):807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- Borda L. J., Shuchleib R., Henry P. D. Hypoxic contraction of isolated canine coronary artery. Mediation by potassium-dependent exocytosis of norepinephrine. Circ Res. 1980 Jun;46(6):870–879. doi: 10.1161/01.res.46.6.870. [DOI] [PubMed] [Google Scholar]
- Brine F., Cornish E. J., Miller R. C. Effects of uptake inhibitors on responses of sheep coronary arteries to catecholamines and sympathetic nerve stimulation. Br J Pharmacol. 1979 Dec;67(4):553–561. doi: 10.1111/j.1476-5381.1979.tb08701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker S. J., Marshall R. J., Parratt J. R., Zeitlin I. J. Does the local myocardial release of prostaglandin E2 or F2alpha contribute to the early consequences of acute myocardial ischaemia? J Mol Cell Cardiol. 1981 Apr;13(4):425–434. doi: 10.1016/0022-2828(81)90284-4. [DOI] [PubMed] [Google Scholar]
- Feletou M., Alya G., Tricoche R., Walden M. Source of calcium and cholinergic contraction of the rat portal vein and the sheep coronary artery. Arch Int Pharmacodyn Ther. 1986 Oct;283(2):254–271. [PubMed] [Google Scholar]
- Folts J. D., Crowell E. B., Jr, Rowe G. G. Platelet aggregation in partially obstructed vessels and its elimination with aspirin. Circulation. 1976 Sep;54(3):365–370. doi: 10.1161/01.cir.54.3.365. [DOI] [PubMed] [Google Scholar]
- Gellai M., Norton J. M., Detar R. Evidence for direct control of coronary vascular tone by oxygen. Circ Res. 1973 Feb;32(2):279–289. doi: 10.1161/01.res.32.2.279. [DOI] [PubMed] [Google Scholar]
- Inoue M., Hifumi K., Kurahashi K., Fujiwara M. Effects of anoxia and ischemia on uptake2 of catecholamines in perfused rat heart. Eur J Pharmacol. 1987 Sep 11;141(2):203–207. doi: 10.1016/0014-2999(87)90264-0. [DOI] [PubMed] [Google Scholar]
- Kalsner S. Coronary artery reactivity in human vessels: some questions and some answers. Fed Proc. 1985 Feb;44(2):321–325. [PubMed] [Google Scholar]
- Kalsner S. The effect of hypoxia on prostaglandin output and on tone in isolated coronary arteries. Can J Physiol Pharmacol. 1977 Aug;55(4):882–887. doi: 10.1139/y77-117. [DOI] [PubMed] [Google Scholar]
- Karmazyn M., Beamish R. E., Dhalla N. S. Involvement of calcium in coronary vasoconstriction due to prolonged hypoxia. Am Heart J. 1984 Feb;107(2):293–297. doi: 10.1016/0002-8703(84)90377-6. [DOI] [PubMed] [Google Scholar]
- Karmazyn M., Horrobin D. F., Oka M., Manku M. S., Ally A. I., Karmali R. A., Morgan R. O., Cunnane S. C. Changes in coronary vascular resistance associated with prolonged hypoxia in isolated rat hearts: a possible role of prostaglandins. Life Sci. 1979 Dec 3;25(23):1991–1999. doi: 10.1016/0024-3205(79)90603-9. [DOI] [PubMed] [Google Scholar]
- Kléber A. G. Extracellular potassium accumulation in acute myocardial ischemia. J Mol Cell Cardiol. 1984 May;16(5):389–394. doi: 10.1016/s0022-2828(84)80610-0. [DOI] [PubMed] [Google Scholar]
- Lewy R. I., Wiener L., Walinsky P., Lefer A. M., Silver M. J., Smith J. B. Thromboxane release during pacing-induced angina pectoris: possible vasoconstrictor influence on the coronary vasculature. Circulation. 1980 Jun;61(6):1165–1171. doi: 10.1161/01.cir.61.6.1165. [DOI] [PubMed] [Google Scholar]
- Pérez J. E., Saffitz J. E., Gutiérrez F. A., Henry P. D. Coronary artery spasm in intact dogs induced by potassium and serotonin. Circ Res. 1983 Apr;52(4):423–431. doi: 10.1161/01.res.52.4.423. [DOI] [PubMed] [Google Scholar]
- Roberts A. M., Messina E. J., Kaley G. Prostacyclin (PGI2) mediates hypoxic relaxation of bovine coronary arterial strips. Prostaglandins. 1981 Apr;21(4):555–569. doi: 10.1016/0090-6980(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Hypoxia releases a vasoconstrictor substance from the canine vascular endothelium. J Physiol. 1985 Jul;364:45–56. doi: 10.1113/jphysiol.1985.sp015728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G., Paul R. J. Two distinct effects of oxygen on vascular tone in isolated porcine coronary arteries. Circ Res. 1985 Jan;56(1):1–10. doi: 10.1161/01.res.56.1.1. [DOI] [PubMed] [Google Scholar]
- Schrör K., Link H. B., Rösen R., Klaus W., Rösen P. Prostacyclin-induced coronary vasodilation. Interactions with adenosine, cyclic AMP and energy charge in the rat heart in vitro. Eur J Pharmacol. 1980 Jun 27;64(4):341–348. doi: 10.1016/0014-2999(80)90242-3. [DOI] [PubMed] [Google Scholar]
- Van Nueten J. M., Van Beek J., Vanhoutte P. M. Effect of Ca2+-entry blockade and serotonergic antagonism on contractions of hypoxic isolated canine coronary arteries. Arch Int Pharmacodyn Ther. 1983 Sep;265(1):172–176. [PubMed] [Google Scholar]
- Yasue H., Touyama M., Shimamoto M., Kato H., Tanaka S. Role of autonomic nervous system in the pathogenesis of Prinzmetal's variant form of angina. Circulation. 1974 Sep;50(3):534–539. doi: 10.1161/01.cir.50.3.534. [DOI] [PubMed] [Google Scholar]
- van Nueten J. M., Vanhoutte P. M. Effect of the Ca2+ antagonist lidoflazine on normoxic and anoxic contractions of canine coronary arterial smooth muscle. Eur J Pharmacol. 1980 Jun 13;64(2-3):173–176. doi: 10.1016/0014-2999(80)90042-4. [DOI] [PubMed] [Google Scholar]


