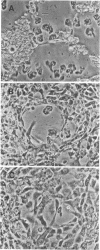
Abstract
The phenothiazine compounds trifluoperazine dihydrochloride and chlorpromazine hydrochloride have in vitro activity against the pathogenic free-living amoebae Naegleria fowleri, Acanthamoeba culbertsoni, and Acanthamoeba polyphaga. Drug concentrations of 10 microM were amoebastatic; concentrations of 50 microM were either amoebastatic or amoebicidal. Concentrations of 100 microM were generally amoebicidal. The mechanism of drug action is unclear. It may reflect sensitivity of amoeba calcium regulatory protein to the phenothiazine compounds or may be due to the lipophilic action of the drugs on the amoeba plasma membrane. Accumulation of these drugs in the central nervous system makes them potentially useful chemotherapeutic agents in humans for treatment of amoebic meningoencephalitis caused by N. fowleri and Acanthamoeba spp.
Full text
PDF
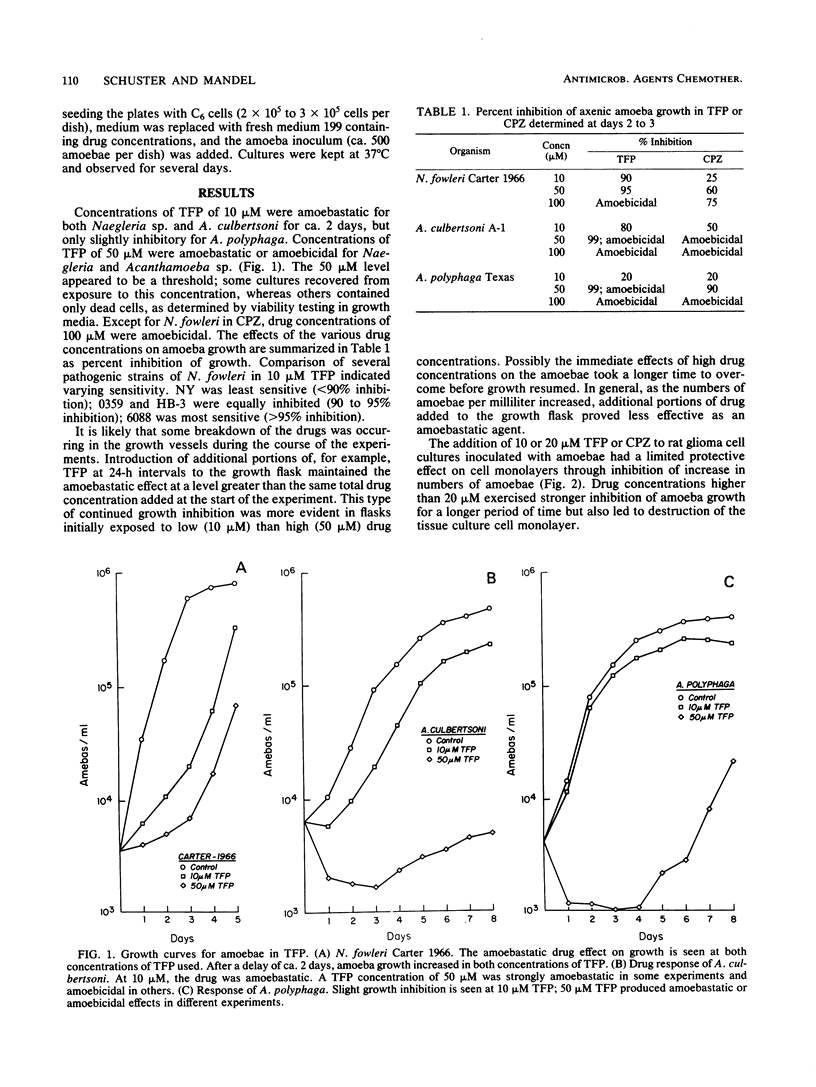
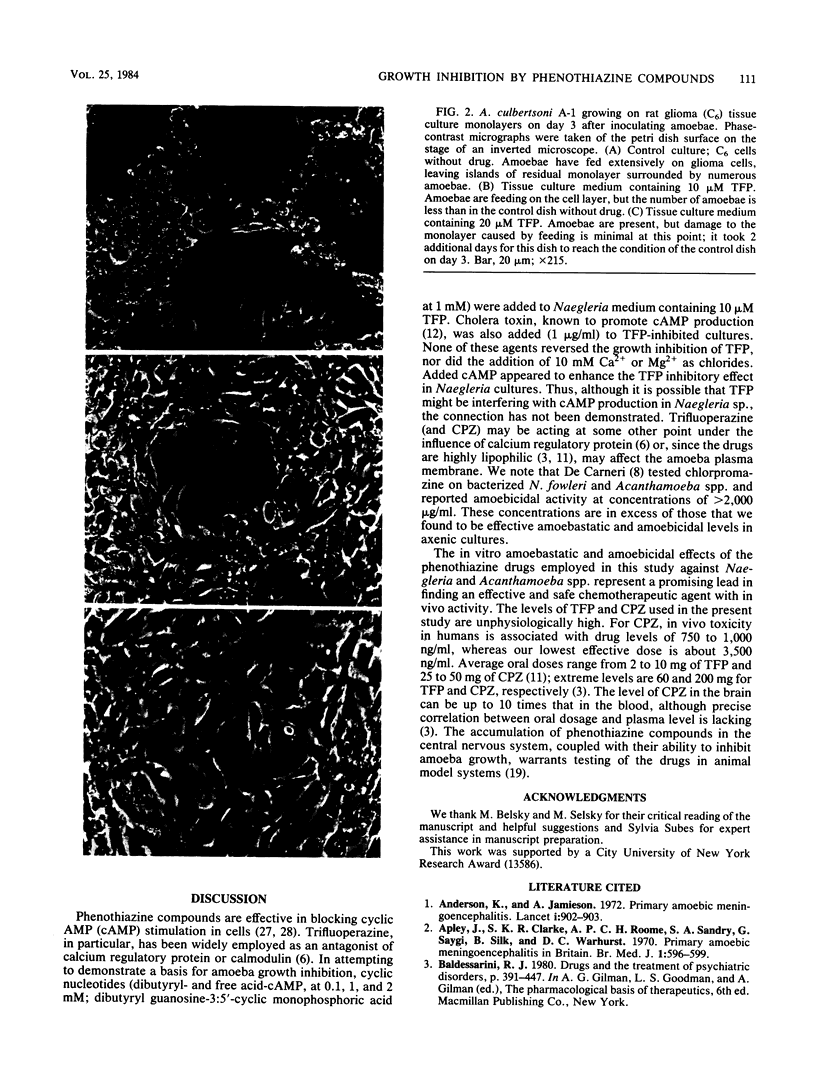

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K., Jamieson A. Primary amoebic meningoencephalitis. Lancet. 1972 Apr 22;1(7756):902–903. doi: 10.1016/s0140-6736(72)90772-6. [DOI] [PubMed] [Google Scholar]
- Apley J., Clarke S. K., Roome A. P., Sandry S. A., Saygi G., Silk B., Warhurst D. C. Primary amoebic meningoencephalitis in Britain. Br Med J. 1970 Mar 7;1(5696):596–599. doi: 10.1136/bmj.1.5696.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. F. Description of a Naegleria sp. isolated from two cases of primary amoebic meningo-encephalitis, and of the experimental pathological changes induced by it. J Pathol. 1970 Apr;100(4):217–244. doi: 10.1002/path.1711000402. [DOI] [PubMed] [Google Scholar]
- Carter R. F. Sensitivity to amphotericin B of a Naegleria sp. isolated from a case of primary amoebic meningoencephalitis. J Clin Pathol. 1969 Jul;22(4):470–474. doi: 10.1136/jcp.22.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin: an overview. Fed Proc. 1982 May;41(7):2253–2257. [PubMed] [Google Scholar]
- De Carneri I. Sensibilita' ai farmaci di amebe del suolo dei generi Hartmannella e Naegleria, agenti eziologici di meningoencefaliti. Riv Parassitol. 1970 Mar;31(1):1–8. [PubMed] [Google Scholar]
- Ferrante A. Comparative sensitivity of Naegleria fowleri to amphotericin B and amphotericin B methyl ester. Trans R Soc Trop Med Hyg. 1982;76(4):476–478. doi: 10.1016/0035-9203(82)90141-9. [DOI] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- John D. T. Primary amebic meningoencephalitis and the biology of Naegleria fowleri. Annu Rev Microbiol. 1982;36:101–123. doi: 10.1146/annurev.mi.36.100182.000533. [DOI] [PubMed] [Google Scholar]
- Jones D. B., Visvesvara G. S., Robinson N. M. Acanthamoeba polyphaga keratitis and Acenthamoeba uveitis associated with fatal meningoencephalitis. Trans Ophthalmol Soc U K. 1975 Jul;95(2):221–232. [PubMed] [Google Scholar]
- Key S. N., 3rd, Green W. R., Willaert E., Stevens A. R., Key S. N., Jr Keratitis due to Acanthamoeba castellani. A clinicopathologic case report. Arch Ophthalmol. 1980 Mar;98(3):475–479. doi: 10.1001/archopht.1980.01020030471005. [DOI] [PubMed] [Google Scholar]
- Lee K. K., Karr S. L., Jr, Wong M. M., Hoeprich P. D. In vitro susceptibilities of Naegleria fowleri strain HB-1 to selected antimicrobial agents, singly and in combination. Antimicrob Agents Chemother. 1979 Aug;16(2):217–220. doi: 10.1128/aac.16.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma P., Willaert E., Juechter K. B., Stevens A. R. A case of keratitis due to Acanthamoeba in New York, New York, and features of 10 cases. J Infect Dis. 1981 May;143(5):662–667. doi: 10.1093/infdis/143.5.662. [DOI] [PubMed] [Google Scholar]
- Martinez A. J., Nelson E. C., Duma R. J. Animal model of human disease. Primary amebic meningoencephalitis, Naegleria meningoencephalitis, CNS protozoal infection. Am J Pathol. 1973 Nov;73(2):545–548. [PMC free article] [PubMed] [Google Scholar]
- Martínez A. J. Is Acanthamoeba encephalitis an opportunistic infection? Neurology. 1980 Jun;30(6):567–574. doi: 10.1212/wnl.30.6.567. [DOI] [PubMed] [Google Scholar]
- Martínez A. J., Sotelo-Avila C., Garcia-Tamayo J., Morón J. T., Willaert E., Stamm W. P. Meningoencephalitis due to Acanthamoeba SP. Pathogenesis and clinico-pathological study. Acta Neuropathol. 1977 Mar 31;37(3):183–191. doi: 10.1007/BF00686877. [DOI] [PubMed] [Google Scholar]
- Nagington J., Richards J. E. Chemotherapeutic compounds and Acanthamoebae from eye infections. J Clin Pathol. 1976 Jul;29(7):648–651. doi: 10.1136/jcp.29.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page F. C. Taxonomic criteria for limax amoebae, with descriptions of 3 new species of Hartmannella and 3 of Vahlkampfia. J Protozool. 1967 Aug;14(3):499–521. doi: 10.1111/j.1550-7408.1967.tb02036.x. [DOI] [PubMed] [Google Scholar]
- Rowan-Kelly B., Ferrante A., Thong Y. H. The chemotherapeutic value of sulphadiazine in treatment of Acanthamoeba meningoencephalitis in mice. Trans R Soc Trop Med Hyg. 1982;76(5):636–638. doi: 10.1016/0035-9203(82)90229-2. [DOI] [PubMed] [Google Scholar]
- Schuster F. L., Rechthand E. In vitro effects of amphotericin B on growth and ultrastructure of the amoeboflagellates Naegleria gruberi and Naegleria fowleri. Antimicrob Agents Chemother. 1975 Nov;8(5):591–605. doi: 10.1128/aac.8.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster F. L., Twomey R. Calcium regulation of flagellation in Naegleria gruberi. J Cell Sci. 1983 Sep;63:311–326. doi: 10.1242/jcs.63.1.311. [DOI] [PubMed] [Google Scholar]
- Seidel J. S., Harmatz P., Visvesvara G. S., Cohen A., Edwards J., Turner J. Successful treatment of primary amebic meningoencephalitis. N Engl J Med. 1982 Feb 11;306(6):346–348. doi: 10.1056/NEJM198202113060607. [DOI] [PubMed] [Google Scholar]
- Shapiro A. P., Sapira J. D., Scheib E. T. Development of bacteriuria in a hypertensive population. A 7-year follow-up study. Ann Intern Med. 1971 Jun;74(6):861–868. doi: 10.7326/0003-4819-74-6-861. [DOI] [PubMed] [Google Scholar]
- Stevens A. R., Shulman S. T., Lansen T. A., Cichon M. J., Willaert E. Primary amoebic meningoencephalitis: a report of two cases and antibiotic and immunologic studies. J Infect Dis. 1981 Feb;143(2):193–199. doi: 10.1093/infdis/143.2.193. [DOI] [PubMed] [Google Scholar]
- Uzunov P., Weiss B. Psychopharmacological agents and the cyclic AMP system of rat brain. Adv Cyclic Nucleotide Res. 1972;1:435–453. [PubMed] [Google Scholar]
- Weiss B., Levin R. M. Mechanism for selectively inhibiting the activation of cyclic nucleotide phosphodiesterase and adenylate cyclase by antipsychotic agents. Adv Cyclic Nucleotide Res. 1978;9:285–303. [PubMed] [Google Scholar]