Abstract
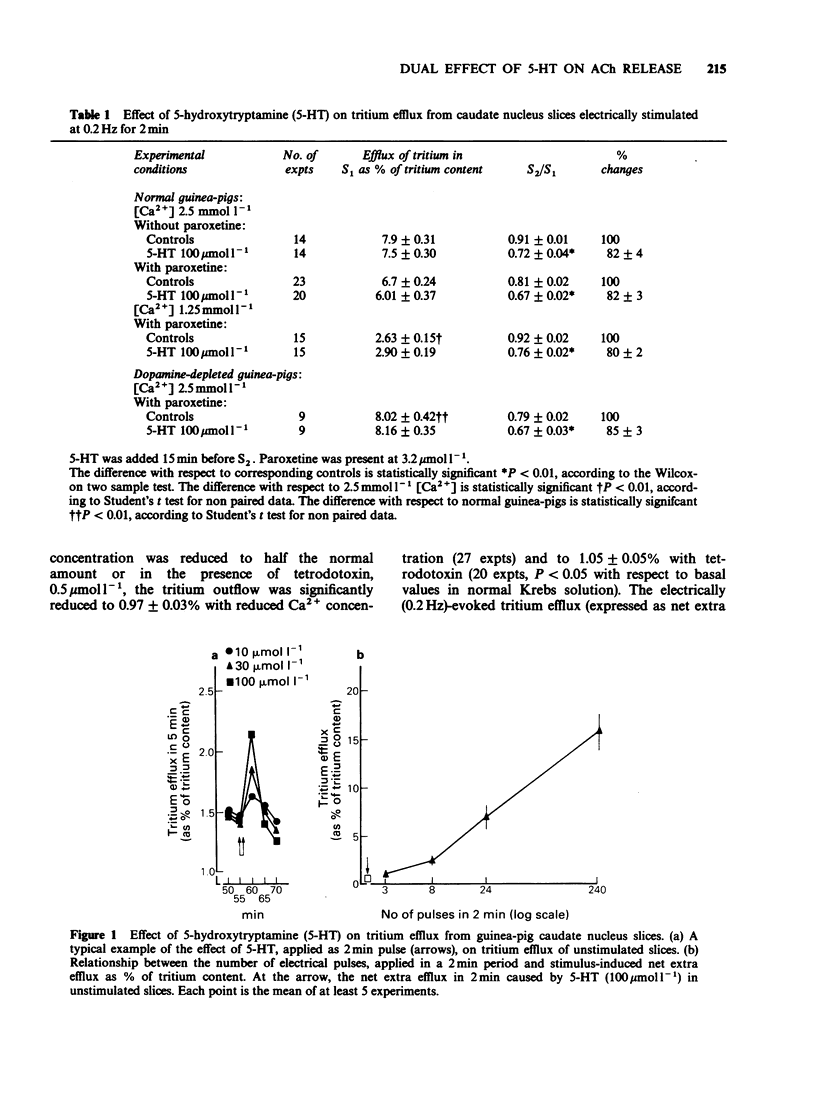
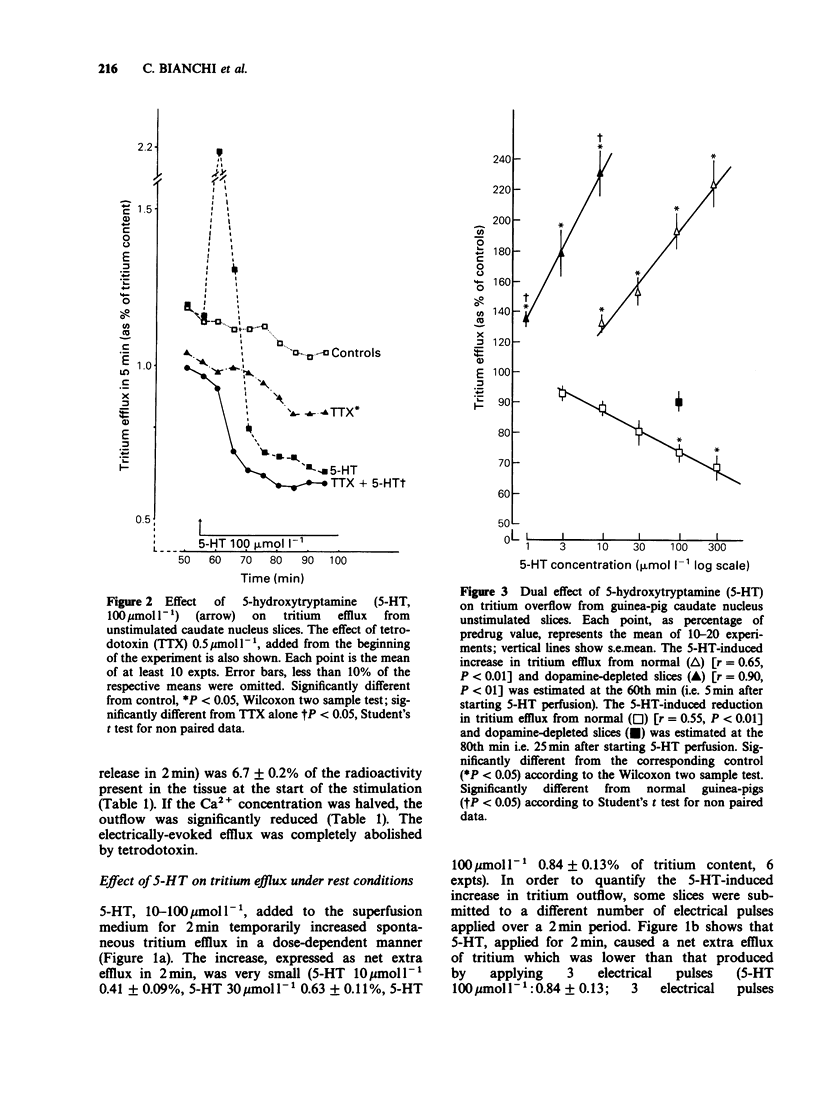
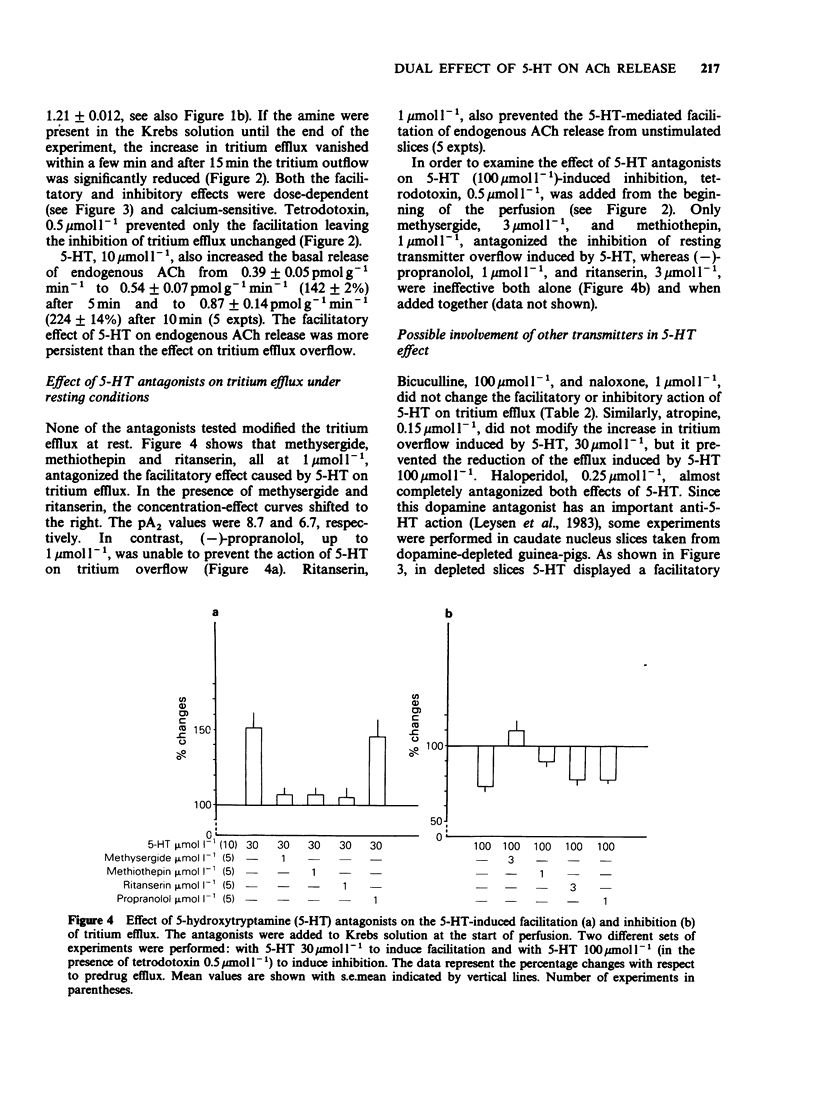
1. The effect of 5-hydroxytryptamine (5-HT) on spontaneous and electrically-evoked tritium efflux was studied in guinea-pig caudate nucleus slices preloaded with [3H]-choline. 2. 5-HT, 10-300 mumol l-1, temporarily increased the spontaneous tritium efflux (as well as the endogenous acetylcholine (ACh) release) and, after 15 min perfusion, inhibited it. The facilitatory effect of 5-HT on spontaneous efflux was increased while the inhibitory effect did not occur in slices taken from dopamine-depleted guinea-pigs. 3. The increase in spontaneous tritium efflux by 5-HT was blocked by methiothepin, methysergide (pA2 8.7) and by the selective 5-HT2 antagonist, ritanserin (pA2 6.7). 4. The inhibition of spontaneous tritium efflux by 5-HT was prevented by methysergide and methiothepin but not by ritanserin and (-)-propranolol. 5. 5-HT, 100 mumol l-1, inhibited the electrically-evoked tritium efflux and this effect was unchanged in dopamine-depleted slices. 6. The inhibition of electrically-evoked tritium efflux by 5-HT was blocked by methiothepin and methysergide but not by (-)-propranolol or ritanserin. 7. These results suggest that 5-HT may exert a rapid and transient (excitatory) and a more prolonged (inhibitory) control over striatal cholinergic neurones.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beani L., Bianchi C., Giacomelli A., Tamberi F. Noradrenaline inhibition of acetylcholine release from guinea-pig brain. Eur J Pharmacol. 1978 Mar 15;48(2):179–193. doi: 10.1016/0014-2999(78)90327-8. [DOI] [PubMed] [Google Scholar]
- Beani L., Bianchi C., Siniscalchi A., Sivilotti L., Tanganelli S., Veratti E. Different approaches to study acetylcholine release: endogenous ACh versus tritium efflux. Naunyn Schmiedebergs Arch Pharmacol. 1984 Dec;328(2):119–126. doi: 10.1007/BF00512060. [DOI] [PubMed] [Google Scholar]
- Bianchi C., Siniscalchi A., Beani L. The influence of 5-hydroxytryptamine on the release of acetylcholine from guinea-pig brain ex vivo and in vitro. Neuropharmacology. 1986 Sep;25(9):1043–1049. doi: 10.1016/0028-3908(86)90200-5. [DOI] [PubMed] [Google Scholar]
- Bianchi C., Tanganelli S., Beani L. Dopamine modulation of acetylcholine release from the guinea-pig brain. Eur J Pharmacol. 1979 Oct 1;58(3):235–246. doi: 10.1016/0014-2999(79)90472-2. [DOI] [PubMed] [Google Scholar]
- Bianchi C., Tanganelli S., Marzola G., Beani L. GABA induced changes in acetylcholine release from slices of guinea-pig brain. Naunyn Schmiedebergs Arch Pharmacol. 1982 Mar;318(4):253–258. doi: 10.1007/BF00501162. [DOI] [PubMed] [Google Scholar]
- Bradley P. B., Engel G., Feniuk W., Fozard J. R., Humphrey P. P., Middlemiss D. N., Mylecharane E. J., Richardson B. P., Saxena P. R. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology. 1986 Jun;25(6):563–576. doi: 10.1016/0028-3908(86)90207-8. [DOI] [PubMed] [Google Scholar]
- Butcher S. H., Butcher L. L., Cho A. K. Modulation of neostriatal acetylcholine in the rat by dopamine and 5-hydroxytryptamine afferents. Life Sci. 1976 Apr 1;18(7):733–743. doi: 10.1016/0024-3205(76)90185-5. [DOI] [PubMed] [Google Scholar]
- Consolo S., Ladinsky H., Forloni G. L., Tirelli A. S., Garattini S. Comparison of the effects of the stereoisomers of fenfluramine on the acetylcholine content of rat striatum, hippocampus and nucleus accumbens. J Pharm Pharmacol. 1980 Mar;32(3):201–203. doi: 10.1111/j.2042-7158.1980.tb12890.x. [DOI] [PubMed] [Google Scholar]
- Euvrard C., Javoy F., Herbet A., Glowinski J. Effect of quipazine, a serotonin-like drug, on striatal cholinergic interneurones. Eur J Pharmacol. 1977 Feb 7;41(3):281–289. doi: 10.1016/0014-2999(77)90321-1. [DOI] [PubMed] [Google Scholar]
- Galarraga E., Bargas J., Martínez-Fong D., Aceves J. Spontaneous synaptic potentials in dopamine-denervated neostriatal neurons. Neurosci Lett. 1987 Oct 29;81(3):351–355. doi: 10.1016/0304-3940(87)90409-5. [DOI] [PubMed] [Google Scholar]
- Gillet G., Ammor S., Fillion G. Serotonin inhibits acetylcholine release from rat striatum slices: evidence for a presynaptic receptor-mediated effect. J Neurochem. 1985 Dec;45(6):1687–1691. doi: 10.1111/j.1471-4159.1985.tb10523.x. [DOI] [PubMed] [Google Scholar]
- Hertting G., Zumstein A., Jackisch R., Hoffmann I., Starke K. Modulation by endogenous dopamine of the release of acetylcholine in the caudate nucleus of the rabbit. Naunyn Schmiedebergs Arch Pharmacol. 1980;315(2):111–117. doi: 10.1007/BF00499253. [DOI] [PubMed] [Google Scholar]
- Kilbinger H., Pfeuffer-Friederich I. Two types of receptors for 5-hydroxytryptamine on the cholinergic nerves of the guinea-pig myenteric plexus. Br J Pharmacol. 1985 Jun;85(2):529–539. doi: 10.1111/j.1476-5381.1985.tb08890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofo-Abayomi A., Lucas P. D. A comparison between atria from control and streptozotocin-diabetic rats: the effects of dietary myoinositol. Br J Pharmacol. 1988 Jan;93(1):3–8. doi: 10.1111/j.1476-5381.1988.tb11399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J., Scatton B. Characterization of the excitatory amino acid receptor-mediated release of [3H]acetylcholine from rat striatal slices. Brain Res. 1982 Dec 2;252(1):77–89. doi: 10.1016/0006-8993(82)90980-5. [DOI] [PubMed] [Google Scholar]
- Maura G., Raiteri M. Cholinergic terminals in rat hippocampus possess 5-HT1B receptors mediating inhibition of acetylcholine release. Eur J Pharmacol. 1986 Oct 7;129(3):333–337. doi: 10.1016/0014-2999(86)90443-7. [DOI] [PubMed] [Google Scholar]
- Pedata F., Antonelli T., Lambertini L., Beani L., Pepeu G. Effect of adenosine, adenosine triphosphate, adenosine deaminase, dipyridamole and aminophylline on acetylcholine release from electrically-stimulated brain slices. Neuropharmacology. 1983 May;22(5):609–614. doi: 10.1016/0028-3908(83)90152-1. [DOI] [PubMed] [Google Scholar]
- Richardson I. W., Szerb J. C. The release of labelled acetylcholine and choline from cerebral cortical slices stimulated electrically. Br J Pharmacol. 1974 Dec;52(4):499–507. doi: 10.1111/j.1476-5381.1974.tb09717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. E. Effect of specific serotonergic lesions on cholinergic neurons in the hippocampus, cortex and striatum. Life Sci. 1983 Jan 24;32(4):345–353. doi: 10.1016/0024-3205(83)90080-2. [DOI] [PubMed] [Google Scholar]
- Vizi E. S., Hársing L. G., Jr, Zsilla G. Evidence of the modulatory role of serotonin in acetylcholine release from striatal interneurons. Brain Res. 1981 May 11;212(1):89–99. doi: 10.1016/0006-8993(81)90035-4. [DOI] [PubMed] [Google Scholar]
- Wikberg J. Release of 3H-acetylcholine from isolated guinea pig ileum. A radiochemical method for studying the release on the cholinergic neurotransmitter in the intestine. Acta Physiol Scand. 1977 Nov;101(3):302–317. doi: 10.1111/j.1748-1716.1977.tb06012.x. [DOI] [PubMed] [Google Scholar]


