Abstract
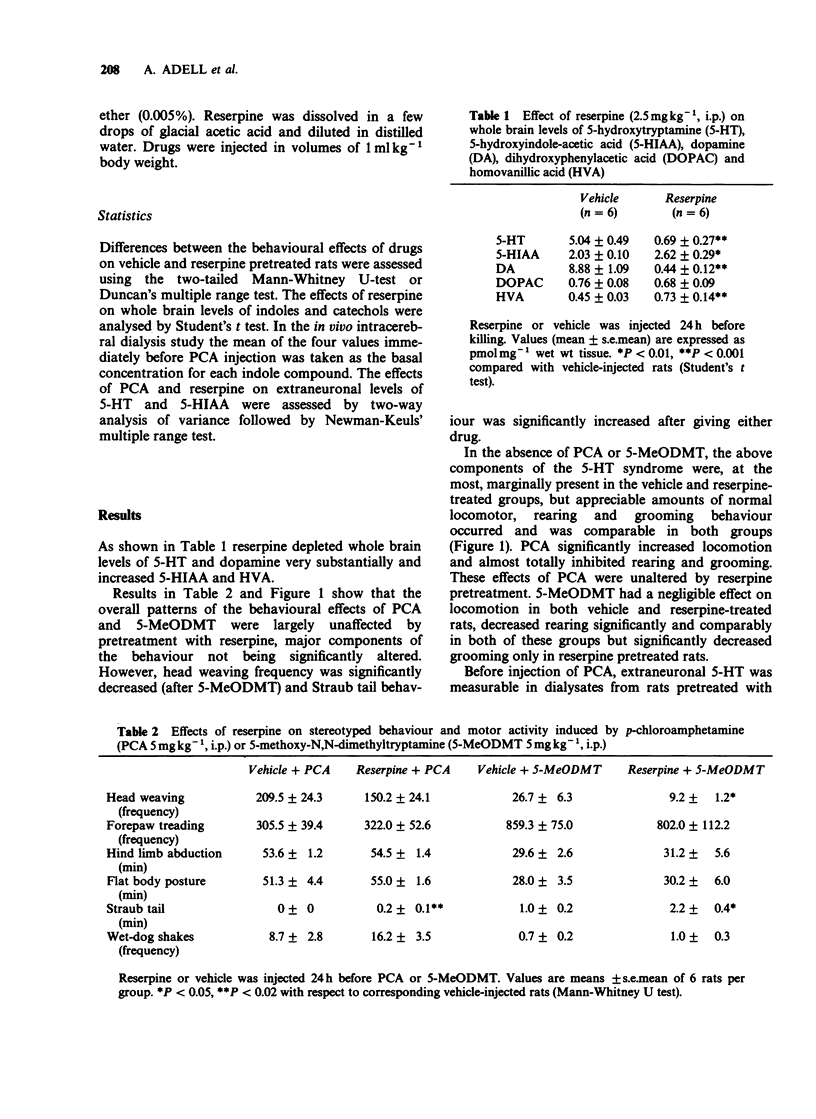
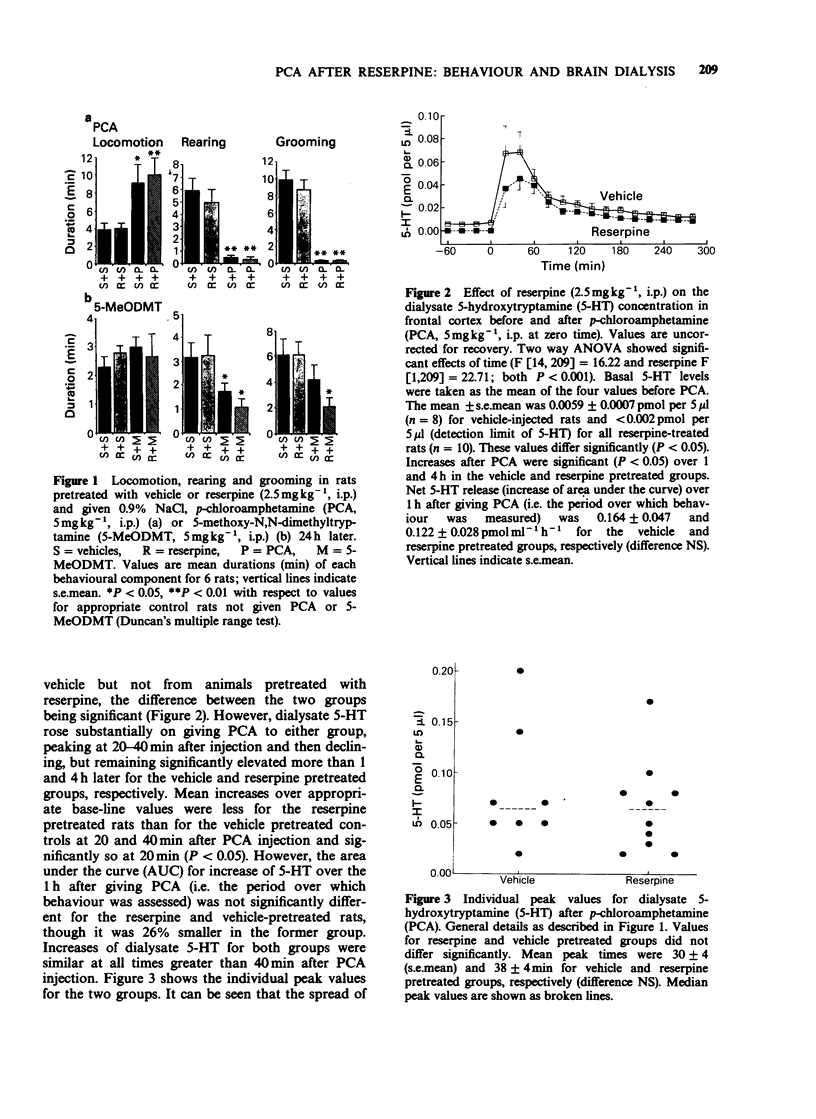
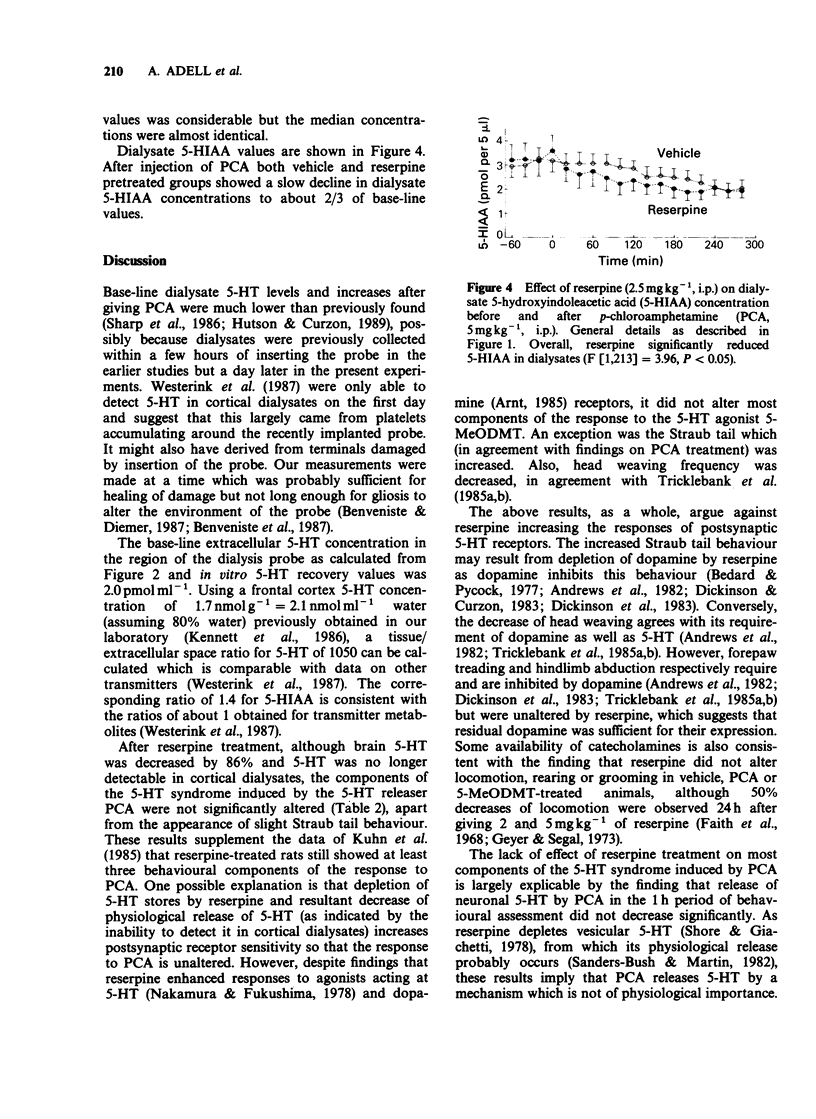
1. Reserpine (2.5 mg kg-1 i.p.) decreased rat brain 5-hydroxytryptamine (5-HT) by 86% 24 h later but most components of the 5-HT-dependent behavioural syndrome induced by p-chloroamphetamine (PCA, 5 mg kg-1 i.p.) or 5-methoxy-N,N-dimethyltryptamine (5-MeODMT, 5 mg kg-1 i.p.) over 1 h after administration were unaffected. However, Straub tail was increased after giving PCA or 5-MeODMT and head weaving was decreased after giving 5-MeODMT. 2. Frontal cortex extracellular 5-HT concentrations of vehicle pretreated rats before injection of PCA, as calculated from dialysate 5-HT concentrations, were about 1/1000th of corresponding brain values. Extracellular 5-hydroxyindoleacetic acid (5-HIAA) and brain values were comparable with each other. Dialysate 5-HT increased after PCA with peak values at 20-40 min. 3. Reserpine pretreatment reduced dialysate 5-HT concentration before PCA was given but the net increase (AUC) over the 1 h after PCA did not differ significantly from that seen in animals pretreated with vehicle. Dialysate 5-HIAA values slowly decreased after PCA injection in both reserpine and vehicle pretreated groups. 4. The results suggest that PCA causes the 5-HT syndrome by releasing 5-HT from the neuronal cytoplasm but that physiological release of 5-HT occurs from vesicular stores.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews C. D., Fernando J. C., Curzon G. Differential involvement of dopamine-containing tracts in 5-hydroxytryptamine-dependent behaviours caused by amphetamine in large doses. Neuropharmacology. 1982 Jan;21(1):63–68. doi: 10.1016/0028-3908(82)90212-x. [DOI] [PubMed] [Google Scholar]
- Arnt J. Behavioural stimulation is induced by separate dopamine D-1 and D-2 receptor sites in reserpine-pretreated but not in normal rats. Eur J Pharmacol. 1985 Jul 11;113(1):79–88. doi: 10.1016/0014-2999(85)90345-0. [DOI] [PubMed] [Google Scholar]
- Bedard P., Pycock C. J. "Wet-dog" shake behaviour in the rat: a possible quantitative model of central 5-hydroxytryptamine activity. Neuropharmacology. 1977 Oct;16(10):663–670. doi: 10.1016/0028-3908(77)90117-4. [DOI] [PubMed] [Google Scholar]
- Benveniste H., Diemer N. H. Cellular reactions to implantation of a microdialysis tube in the rat hippocampus. Acta Neuropathol. 1987;74(3):234–238. doi: 10.1007/BF00688186. [DOI] [PubMed] [Google Scholar]
- Benveniste H., Drejer J., Schousboe A., Diemer N. H. Regional cerebral glucose phosphorylation and blood flow after insertion of a microdialysis fiber through the dorsal hippocampus in the rat. J Neurochem. 1987 Sep;49(3):729–734. doi: 10.1111/j.1471-4159.1987.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Butcher S. P., Fairbrother I. S., Kelly J. S., Arbuthnott G. W. Amphetamine-induced dopamine release in the rat striatum: an in vivo microdialysis study. J Neurochem. 1988 Feb;50(2):346–355. doi: 10.1111/j.1471-4159.1988.tb02919.x. [DOI] [PubMed] [Google Scholar]
- Curzon G., Fernando J. C., Marsden C. A. 5-Hydroxytryptamine: the effects of impaired synthesis on its metabolism and release in rat. Br J Pharmacol. 1978 Aug;63(4):627–634. doi: 10.1111/j.1476-5381.1978.tb17275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson S. L., Curzon G. Roles of dopamine and 5-hydroxytryptamine in stereotyped and non-stereotyped behaviour. Neuropharmacology. 1983 Jul;22(7):805–812. doi: 10.1016/0028-3908(83)90124-7. [DOI] [PubMed] [Google Scholar]
- Dickinson S. L., Jackson A., Curzon G. Effect of apomorphine on behaviour induced by 5-methoxy-N, N-dimethyl tryptamine: three different scoring methods give three different conclusions. Psychopharmacology (Berl) 1983;80(2):196–197. doi: 10.1007/BF00427970. [DOI] [PubMed] [Google Scholar]
- Donohoe T. P., Hutson P. H., Curzon G. Blockade of dopamine receptors explains the lack of 5-HT stereotypy on treatment with the putative 5-HT1A agonist LY165163. Psychopharmacology (Berl) 1987;93(1):82–86. doi: 10.1007/BF02439591. [DOI] [PubMed] [Google Scholar]
- Dourish C. T., Hutson P. H., Curzon G. Low doses of the putative serotonin agonist 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) elicit feeding in the rat. Psychopharmacology (Berl) 1985;86(1-2):197–204. doi: 10.1007/BF00431709. [DOI] [PubMed] [Google Scholar]
- Faith M. E., Young L. D., Grabarits F., Harvey J. A. Differences in the duration of reserpine action in the rat depending on the measure employed. Int J Neuropharmacol. 1968 Nov;7(6):575–585. doi: 10.1016/0028-3908(68)90068-3. [DOI] [PubMed] [Google Scholar]
- Fernando J. C., Curzon G. Behavioural responses to drugs releasing 5-hydroxytryptamine and catecholamines: effects of treatments altering precursor concentrations in brain. Neuropharmacology. 1981 Feb;20(2):115–122. doi: 10.1016/0028-3908(81)90193-3. [DOI] [PubMed] [Google Scholar]
- Geyer M. A., Segal D. S. Differential effects of reserpine and alpha-methyl-P-tyrosine on norepinephrine and dopamine induced behavioral activity. Psychopharmacologia. 1973 Mar 16;29(2):131–140. doi: 10.1007/BF00422645. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith D. G. Studies in vivo on the relationship between brain tryptophan, brain 5-HT synthesis and hyperactivity in rats treated with a monoamine oxidase inhibitor and L-tryptophan. J Neurochem. 1971 Jun;18(6):1053–1066. doi: 10.1111/j.1471-4159.1971.tb12034.x. [DOI] [PubMed] [Google Scholar]
- Hutson P. H., Sarna G. S., Kantamaneni B. D., Curzon G. Monitoring the effect of a tryptophan load on brain indole metabolism in freely moving rats by simultaneous cerebrospinal fluid sampling and brain dialysis. J Neurochem. 1985 Apr;44(4):1266–1273. doi: 10.1111/j.1471-4159.1985.tb08753.x. [DOI] [PubMed] [Google Scholar]
- Jacobs B. L. An animal behavior model for studying central serotonergic synapses. Life Sci. 1976 Sep 15;19(6):777–785. doi: 10.1016/0024-3205(76)90303-9. [DOI] [PubMed] [Google Scholar]
- Kennett G. A., Chaouloff F., Marcou M., Curzon G. Female rats are more vulnerable than males in an animal model of depression: the possible role of serotonin. Brain Res. 1986 Sep 24;382(2):416–421. doi: 10.1016/0006-8993(86)91355-7. [DOI] [PubMed] [Google Scholar]
- Kennett G. A., Dickinson S. L., Curzon G. Enhancement of some 5-HT-dependent behavioural responses following repeated immobilization in rats. Brain Res. 1985 Mar 25;330(2):253–263. doi: 10.1016/0006-8993(85)90684-5. [DOI] [PubMed] [Google Scholar]
- Kuhn D. M., Wolf W. A., Youdim M. B. 5-Hydroxytryptamine release in vivo from a cytoplasmic pool: studies on the 5-HT behavioural syndrome in reserpinized rats. Br J Pharmacol. 1985 Jan;84(1):121–129. [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Fukushima H. Effects of reserpine, para-chlorophenylalanine, 5,6-dihydroxytryptamine and fludiazepam on the head twitches induced by 5-hydroxytryptamine or 5-methoxytryptamine in mice. J Pharm Pharmacol. 1978 Apr;30(4):254–256. doi: 10.1111/j.2042-7158.1978.tb13219.x. [DOI] [PubMed] [Google Scholar]
- Niddam R., Arbilla S., Scatton B., Dennis T., Langer S. Z. Amphetamine induced release of endogenous dopamine in vitro is not reduced following pretreatment with reserpine. Naunyn Schmiedebergs Arch Pharmacol. 1985 Apr;329(2):123–127. doi: 10.1007/BF00501200. [DOI] [PubMed] [Google Scholar]
- Ross S. B., Kelder D. Efflux of 5-hydroxytryptamine from synaptosomes of rat cerebral cortex. Acta Physiol Scand. 1977 Jan;99(1):27–36. doi: 10.1111/j.1748-1716.1977.tb10348.x. [DOI] [PubMed] [Google Scholar]
- Sharp T., Zetterström T., Christmanson L., Ungerstedt U. p-Chloroamphetamine releases both serotonin and dopamine into rat brain dialysates in vivo. Neurosci Lett. 1986 Dec 23;72(3):320–324. doi: 10.1016/0304-3940(86)90534-3. [DOI] [PubMed] [Google Scholar]
- Shields P. J., Eccleston D. Effects of electrical stimulation of rat midbrain on 5-hydroxytryptamine synthesis as determined by a sensitive radioisotope method. J Neurochem. 1972 Feb;19(2):265–272. doi: 10.1111/j.1471-4159.1972.tb01336.x. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S., Drust E. G., Connor J. D. Specificity of a rat behavioral model for serotonin receptor activation. J Pharmacol Exp Ther. 1978 Aug;206(2):339–347. [PubMed] [Google Scholar]
- Smith L. M., Peroutka S. J. Differential effects of 5-hydroxytryptamine1a selective drugs on the 5-HT behavioral syndrome. Pharmacol Biochem Behav. 1986 Jun;24(6):1513–1519. doi: 10.1016/0091-3057(86)90477-6. [DOI] [PubMed] [Google Scholar]
- Tricklebank M. D., Forler C., Fozard J. R. The involvement of subtypes of the 5-HT1 receptor and of catecholaminergic systems in the behavioural response to 8-hydroxy-2-(di-n-propylamino)tetralin in the rat. Eur J Pharmacol. 1984 Nov 13;106(2):271–282. doi: 10.1016/0014-2999(84)90714-3. [DOI] [PubMed] [Google Scholar]
- Tricklebank M. D., Forler C., Middlemiss D. N., Fozard J. R. Subtypes of the 5-HT receptor mediating the behavioural responses to 5-methoxy-N,N-dimethyltryptamine in the rat. Eur J Pharmacol. 1985 Oct 29;117(1):15–24. doi: 10.1016/0014-2999(85)90467-4. [DOI] [PubMed] [Google Scholar]
- Trulson M. E., Jacobs B. L. Behavioral evidence for the rapid release of CNS serotonin by PCA and fenfluramine. Eur J Pharmacol. 1976 Mar;36(1):149–154. doi: 10.1016/0014-2999(76)90266-1. [DOI] [PubMed] [Google Scholar]
- Westerink B. H., Damsma G., Rollema H., De Vries J. B., Horn A. S. Scope and limitations of in vivo brain dialysis: a comparison of its application to various neurotransmitter systems. Life Sci. 1987 Oct 12;41(15):1763–1776. doi: 10.1016/0024-3205(87)90695-3. [DOI] [PubMed] [Google Scholar]


