Abstract
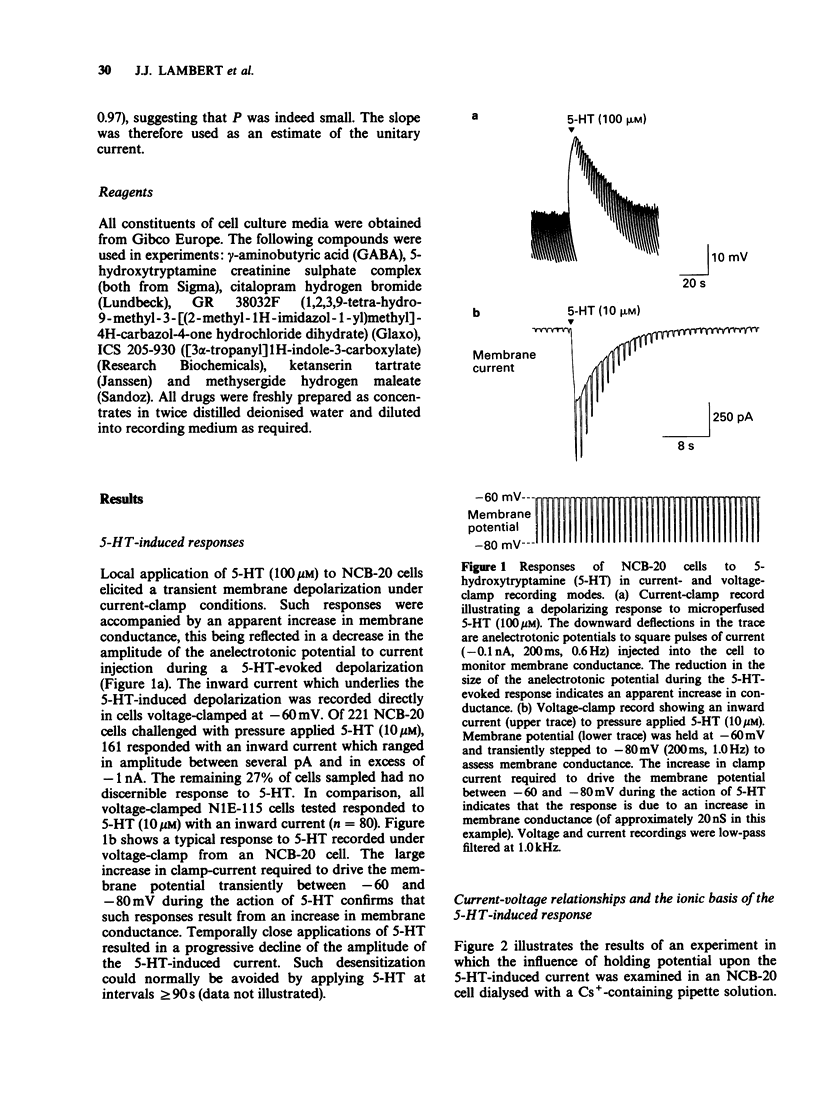
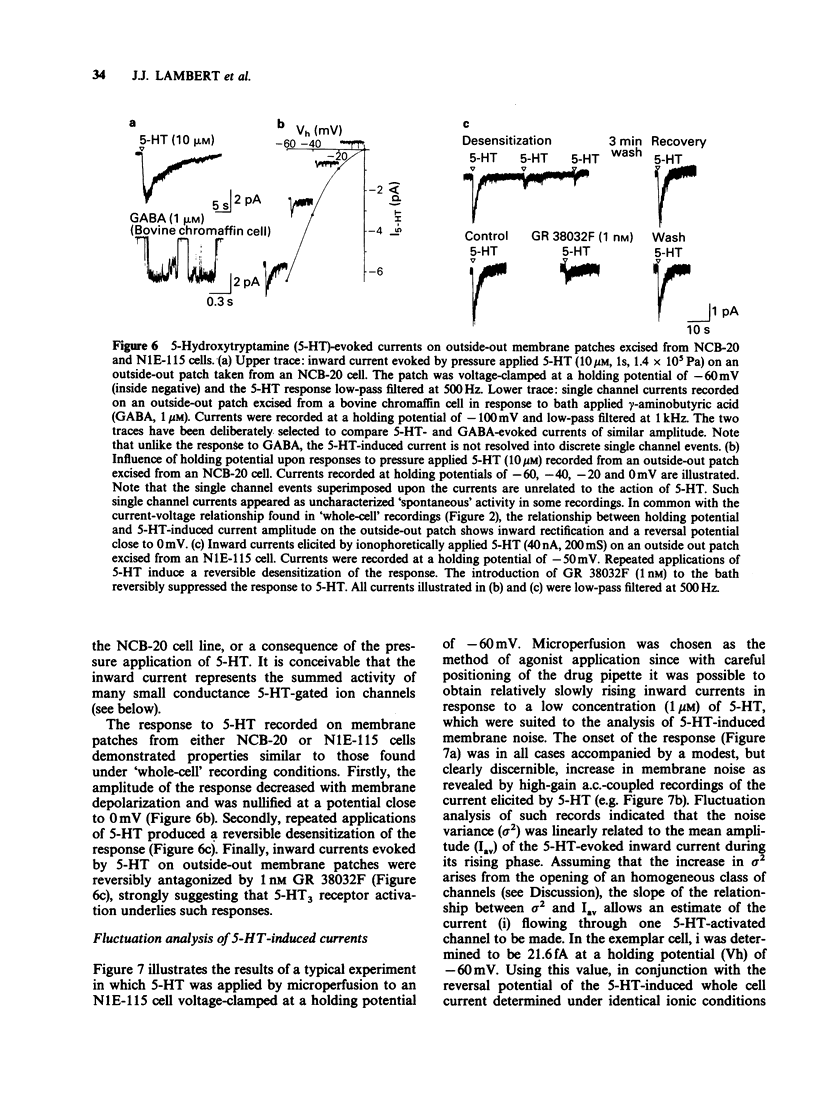
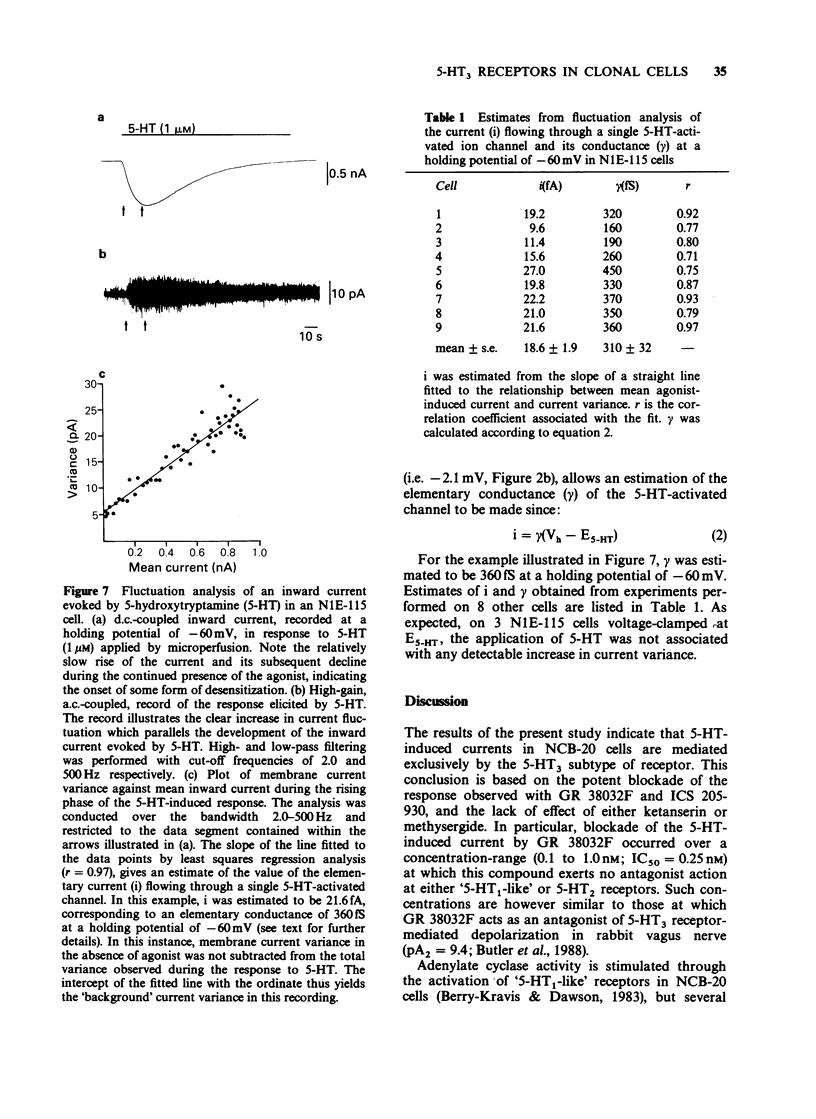
1 The characteristics of transmembrane currents evoked by 5-hydroxytryptamine (5-HT) in the neuroblastoma x Chinese hamster brain cell line NCB-20 and neuroblastoma clonal cell line N1E-115 have been studied under voltage-clamp conditions by the whole-cell recording and outside-out membrane patch modes of the patch-clamp technique. 2 In 73% of NCB-20 cells examined (n = 221), and all N1E-115 cells studied (n = 80), 5-HT (10 microM) elicited a transient inward current at negative holding potentials, this being associated with an increase in membrane conductance. In both cell lines responses to 5-HT reversed in sign at a potential of approximately -2 mV and demonstrated inward rectification. 3 The reversal potential of 5-HT-induced currents (E5-HT) recorded from either NCB-20 or N1E-115 cells was unaffected by total replacement of internal K+ by Cs+. In N1E-115 cells, reducing internal K+ concentration from 140 to 20 mM produced a positive shift in E5-HT of approximately 28 mV, whereas reducing external Na+ from 143 to 20 mM was associated with a negative shift in E5-HT of about 37 mV. A large reduction in internal Cl- concentration (from 144 to 6 mM) had little effect on E5-HT. 4 5-HT-induced currents of NCB-20 cells were unaffected by methysergide (1 microM) or ketanserin (1 microM), but were reversibly antagonized by GR38032F (0.1-1.0 nM) with an IC50 of 0.25 nM. GR 38032F (0.3 nM) reduced 5-HT-induced currents in N1E-115 cells to approximately 26% of their control value. 5 On outside-out membrane patches excised from both NCB-20 and N1E-115 cells, 5-HT induced small inward currents which could not be clearly resolved into discrete single channel events. Such responses were: (i) reversibly antagonized by GR 38032F (1 nM) (ii) reversed in sign at 0 mV, and (iii) subject to desensitization. 6 Fluctuation analysis of inward currents evoked by 5-HT (1 microM) in N1E-115 cells suggests that 5-HT gates a channel with a conductance of approximately 310fS. Such a relatively small conductance could readily explain why the response of outside-out membrane patches to 5-HT cannot at present be resolved into clear single channel events.
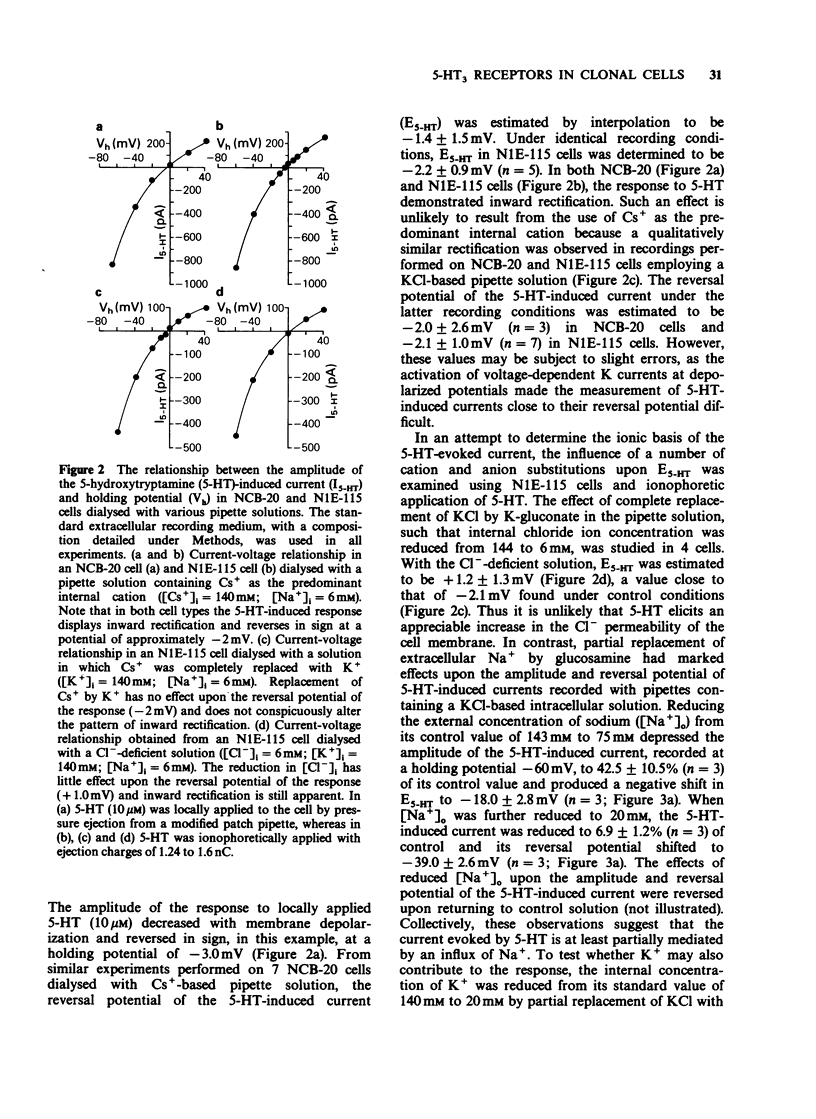
Full text
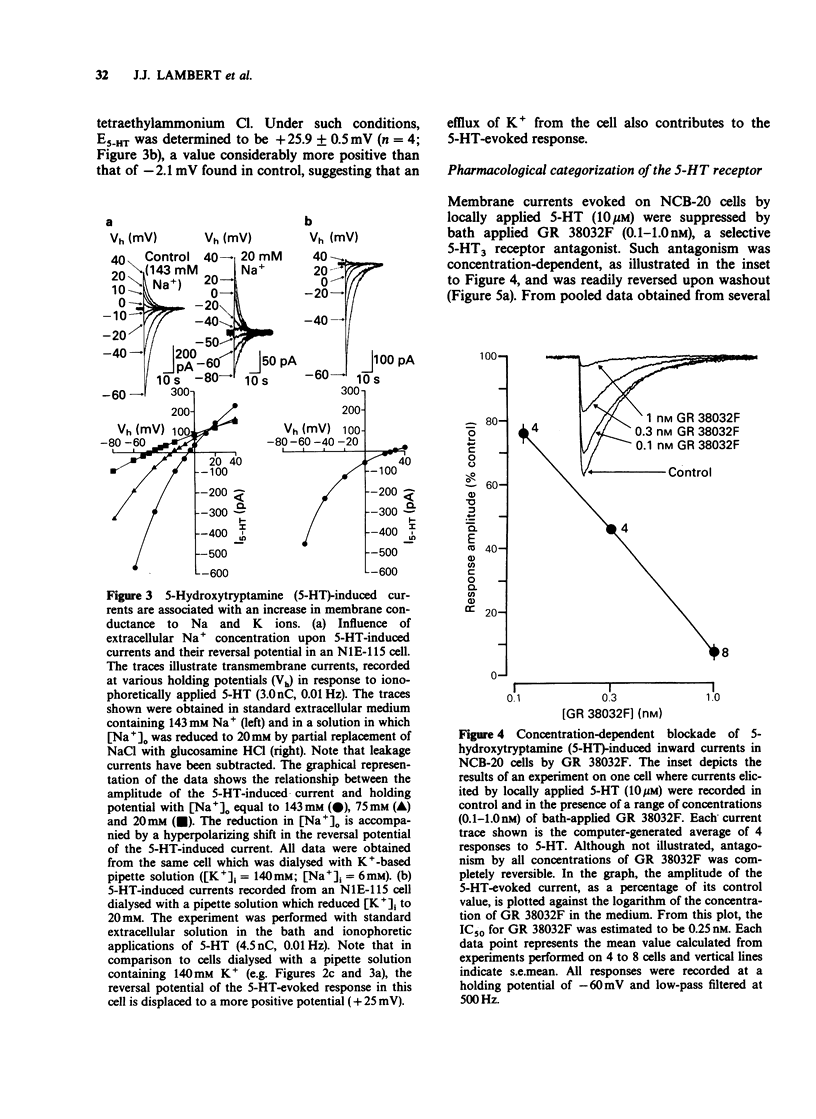
PDF


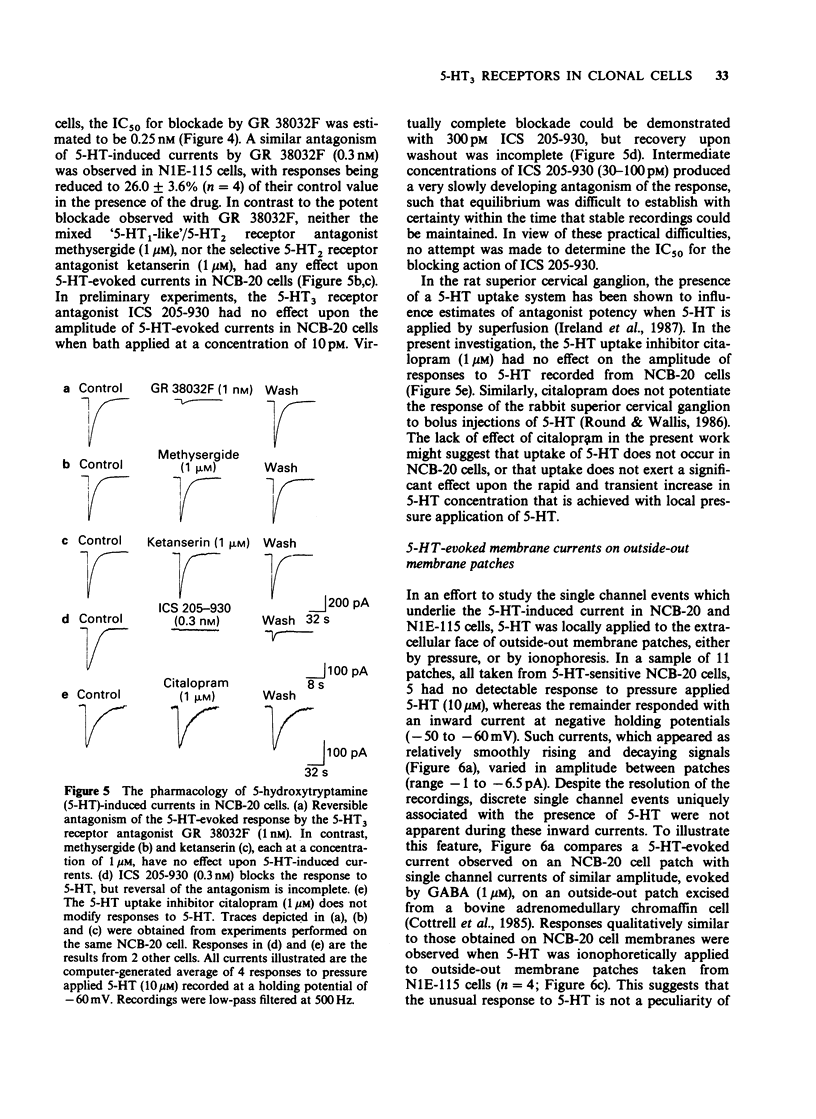





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Bregestovski P., Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988 May;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. Quisqualate- and kainate-activated channels in mouse central neurones in culture. J Physiol. 1988 May;399:227–245. doi: 10.1113/jphysiol.1988.sp017077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes N. M., Costall B., Naylor R. J. [3H]zacopride: ligand for the identification of 5-HT3 recognition sites. J Pharm Pharmacol. 1988 Aug;40(8):548–551. doi: 10.1111/j.2042-7158.1988.tb05300.x. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E., Freedman S. B., Dawson G. Specific receptor-mediated inhibition of cyclic AMP synthesis by dopamine in a neuroblastoma X brain hybrid cell line NCB-20. J Neurochem. 1984 Aug;43(2):413–420. doi: 10.1111/j.1471-4159.1984.tb00917.x. [DOI] [PubMed] [Google Scholar]
- Blandina P., Goldfarb J., Green J. P. Activation of a 5-HT3 receptor releases dopamine from rat striatal slice. Eur J Pharmacol. 1988 Oct 18;155(3):349–350. doi: 10.1016/0014-2999(88)90528-6. [DOI] [PubMed] [Google Scholar]
- Bradley P. B., Engel G., Feniuk W., Fozard J. R., Humphrey P. P., Middlemiss D. N., Mylecharane E. J., Richardson B. P., Saxena P. R. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology. 1986 Jun;25(6):563–576. doi: 10.1016/0028-3908(86)90207-8. [DOI] [PubMed] [Google Scholar]
- Butler A., Hill J. M., Ireland S. J., Jordan C. C., Tyers M. B. Pharmacological properties of GR38032F, a novel antagonist at 5-HT3 receptors. Br J Pharmacol. 1988 Jun;94(2):397–412. doi: 10.1111/j.1476-5381.1988.tb11542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell G. A., Lambert J. J., Peters J. A. Modulation of GABAA receptor activity by alphaxalone. Br J Pharmacol. 1987 Mar;90(3):491–500. doi: 10.1111/j.1476-5381.1987.tb11198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Howe J. R., Ogden D. C. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. J Physiol. 1988 Jun;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Ogden D. C. Ion channels activated by L-glutamate and GABA in cultured cerebellar neurons of the rat. Proc R Soc Lond B Biol Sci. 1985 May 22;224(1236):367–373. doi: 10.1098/rspb.1985.0038. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Iontophoretic application of acetylcholine: advantages of high resistance micropipettes in connection with an electronic current pump. Pflugers Arch. 1974 Apr 22;348(3):263–272. doi: 10.1007/BF00587417. [DOI] [PubMed] [Google Scholar]
- Dukes I. D., Vaughan Williams E. M. Electrophysiological effects of alpha-adrenoceptor antagonists in rabbit sino-atrial node, cardiac Purkinje cells and papillary muscles. Br J Pharmacol. 1984 Oct;83(2):419–426. doi: 10.1111/j.1476-5381.1984.tb16502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozard J. R. MDL 72222: a potent and highly selective antagonist at neuronal 5-hydroxytryptamine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1984 May;326(1):36–44. doi: 10.1007/BF00518776. [DOI] [PubMed] [Google Scholar]
- Fozard J. R. Neuronal 5-HT receptors in the periphery. Neuropharmacology. 1984 Dec;23(12B):1473–1486. doi: 10.1016/0028-3908(84)90091-1. [DOI] [PubMed] [Google Scholar]
- Friend C., Freedman H. A. Effects and possible mechanism of action of dimethylsulfoxide on Friend cell differentiation. Biochem Pharmacol. 1978 May 1;27(9):1309–1313. doi: 10.1016/0006-2952(78)90112-0. [DOI] [PubMed] [Google Scholar]
- Guharay F., Ramsey R. L., Usherwood P. N. 5-Hydroxytryptamine-activated single-channel currents recorded from murine neuroblastoma cells. Brain Res. 1985 Aug 12;340(2):325–332. doi: 10.1016/0006-8993(85)90929-1. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Higashi H., Nishi S. 5-Hydroxytryptamine receptors of visceral primary afferent neurones on rabbit nodose ganglia. J Physiol. 1982 Feb;323:543–567. doi: 10.1113/jphysiol.1982.sp014091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D., Neijt H. C. Identification of serotonin 5-HT3 recognition sites by radioligand binding in NG108-15 neuroblastoma-glioma cells. Eur J Pharmacol. 1987 Nov 10;143(2):291–292. doi: 10.1016/0014-2999(87)90547-4. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Neijt H. C. Identification of serotonin 5-HT3 recognition sites in membranes of N1E-115 neuroblastoma cells by radioligand binding. Mol Pharmacol. 1988 Mar;33(3):303–309. [PubMed] [Google Scholar]
- Ireland S. J., Straughan D. W., Tyers M. B. Influence of 5-hydroxytryptamine uptake on the apparent 5-hydroxytryptamine antagonist potency of metoclopramide in the rat isolated superior cervical ganglion. Br J Pharmacol. 1987 Jan;90(1):151–160. doi: 10.1111/j.1476-5381.1987.tb16835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Jones B. J., Costall B., Domeney A. M., Kelly M. E., Naylor R. J., Oakley N. R., Tyers M. B. The potential anxiolytic activity of GR38032F, a 5-HT3-receptor antagonist. Br J Pharmacol. 1988 Apr;93(4):985–993. doi: 10.1111/j.1476-5381.1988.tb11489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato E., Narahashi T. Characteristics of the electrical response to dopamine in neuroblastoma cells. J Physiol. 1982 Dec;333:213–226. doi: 10.1113/jphysiol.1982.sp014450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick G. J., Jones B. J., Tyers M. B. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987 Dec 24;330(6150):746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- Kimhi Y., Palfrey C., Spector I., Barak Y., Littauer U. Z. Maturation of neuroblastoma cells in the presence of dimethylsulfoxide. Proc Natl Acad Sci U S A. 1976 Feb;73(2):462–466. doi: 10.1073/pnas.73.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermot J., Higashida H., Wilson S. P., Matsuzawa H., Minna J., Nirenberg M. Adenylate cyclase and acetylcholine release regulated by separate serotonin receptors of somatic cell hybrids. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1135–1139. doi: 10.1073/pnas.76.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minna J. D., Yavelow J., Coon H. G. Expression of phenotypes in hybrid somatic cells derived from the nervous system. Genetics. 1975 Jun;79 (Suppl):373–383. [PubMed] [Google Scholar]
- Neijt H. C., Vijverberg H. P., Van den Bercken J. The dopamine response in mouse neuroblastoma cells is mediated by serotonin 5HT3 receptors. Eur J Pharmacol. 1986 Aug 15;127(3):271–274. doi: 10.1016/0014-2999(86)90374-2. [DOI] [PubMed] [Google Scholar]
- Neijt H. C., te Duits I. J., Vijverberg H. P. Pharmacological characterization of serotonin 5-HT3 receptor-mediated electrical response in cultured mouse neuroblastoma cells. Neuropharmacology. 1988 Mar;27(3):301–307. doi: 10.1016/0028-3908(88)90048-2. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Hamik A. [3H]quipazine labels 5-HT3 recognition sites in rat cortical membranes. Eur J Pharmacol. 1988 Mar 29;148(2):297–299. doi: 10.1016/0014-2999(88)90579-1. [DOI] [PubMed] [Google Scholar]
- Peters J. A., Hales T. G., Lambert J. J. Divalent cations modulate 5-HT3 receptor-induced currents in N1E-115 neuroblastoma cells. Eur J Pharmacol. 1988 Jul 14;151(3):491–495. doi: 10.1016/0014-2999(88)90550-x. [DOI] [PubMed] [Google Scholar]
- Richardson B. P., Engel G., Donatsch P., Stadler P. A. Identification of serotonin M-receptor subtypes and their specific blockade by a new class of drugs. Nature. 1985 Jul 11;316(6024):126–131. doi: 10.1038/316126a0. [DOI] [PubMed] [Google Scholar]
- Round A., Wallis D. I. The depolarizing action of 5-hydroxytryptamine on rabbit vagal afferent and sympathetic neurones in vitro and its selective blockade by ICS 205-930. Br J Pharmacol. 1986 Jun;88(2):485–494. doi: 10.1111/j.1476-5381.1986.tb10227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis D. I., North R. A. The action of 5-hydroxytryptamine on single neurones of the rabbit superior cervical ganglion. Neuropharmacology. 1978 Dec;17(12):1023–1028. doi: 10.1016/0028-3908(78)90028-x. [DOI] [PubMed] [Google Scholar]
- Wallis D. I., Woodward B. Membrane potential changes induced by 5-hydroxytryptamine in the rabbit superior cervical ganglion. Br J Pharmacol. 1975 Oct;55(2):199–212. doi: 10.1111/j.1476-5381.1975.tb07629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis D. Neuronal 5-hydroxytryptamine receptors outside the central nervous system. Life Sci. 1981 Dec 7;29(23):2345–2355. doi: 10.1016/0024-3205(81)90470-7. [DOI] [PubMed] [Google Scholar]
- Yakel J. L., Jackson M. B. 5-HT3 receptors mediate rapid responses in cultured hippocampus and a clonal cell line. Neuron. 1988 Sep;1(7):615–621. doi: 10.1016/0896-6273(88)90111-0. [DOI] [PubMed] [Google Scholar]
- Yakel J. L., Trussell L. O., Jackson M. B. Three serotonin responses in cultured mouse hippocampal and striatal neurons. J Neurosci. 1988 Apr;8(4):1273–1285. doi: 10.1523/JNEUROSCI.08-04-01273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoffe J. R., Borchardt R. T. Characterization of serotonin uptake in cultured neuroblastoma cells. Difference between differentiated and nondifferentiated cells. Mol Pharmacol. 1982 Mar;21(2):362–367. [PubMed] [Google Scholar]
- Yoffe J. R., Borchardt R. T. Characterization of serotonin uptake in cultured pheochromocytoma cells. Comparison with norepinephrine uptake. Mol Pharmacol. 1982 Mar;21(2):368–373. [PubMed] [Google Scholar]



