Abstract
1. The effects of continuous intravenous infusion of noradrenaline (0.01 and 0.1 microgram kg-1 h-1) were studied in both the infused lateral saphenous vein and the contralateral saphenous vein of normal dogs. Noradrenaline, saline, noradrenaline + desipramine or noradrenaline + superoxide dismutase were infused using Alzet osmotic minipumps. 2. After a 5 day infusion period, the noradrenaline content in plasma and in both saphenous veins was determined, and the venous tissues submitted to light microscope morphometry and ultrastructural study and used for the determination of their O-methylation capacity (with [3H]-isoprenaline as a substrate). 3. Noradrenaline caused dose-dependent damage to the sympathetic nerve endings of the lateral saphenous veins. Concomitant changes in extraneuronal structure and function were observed (hypertrophy of smooth muscle cells, nuclear dysmorphy, thickening of the vessel wall, impairment in O-methylation capacity). 4. Desipramine and superoxide dismutase prevented or reduced the effects of noradrenaline on both the morphological and the biochemical parameters; the protection afforded by superoxide dismutase was more marked than that by desipramine. 5. It is concluded that moderately high doses of noradrenaline exert a 6-hydroxydopamine-like effect and that this chemical sympathectomy is partially or totally prevented by desipramine or superoxide dismutase. The data suggest that a substance derived from noradrenaline, in the formation of which free oxygen radicals are involved and which is subject to neuronal uptake, is the chemical entity responsible for the neurotoxic effect observed.
Full text
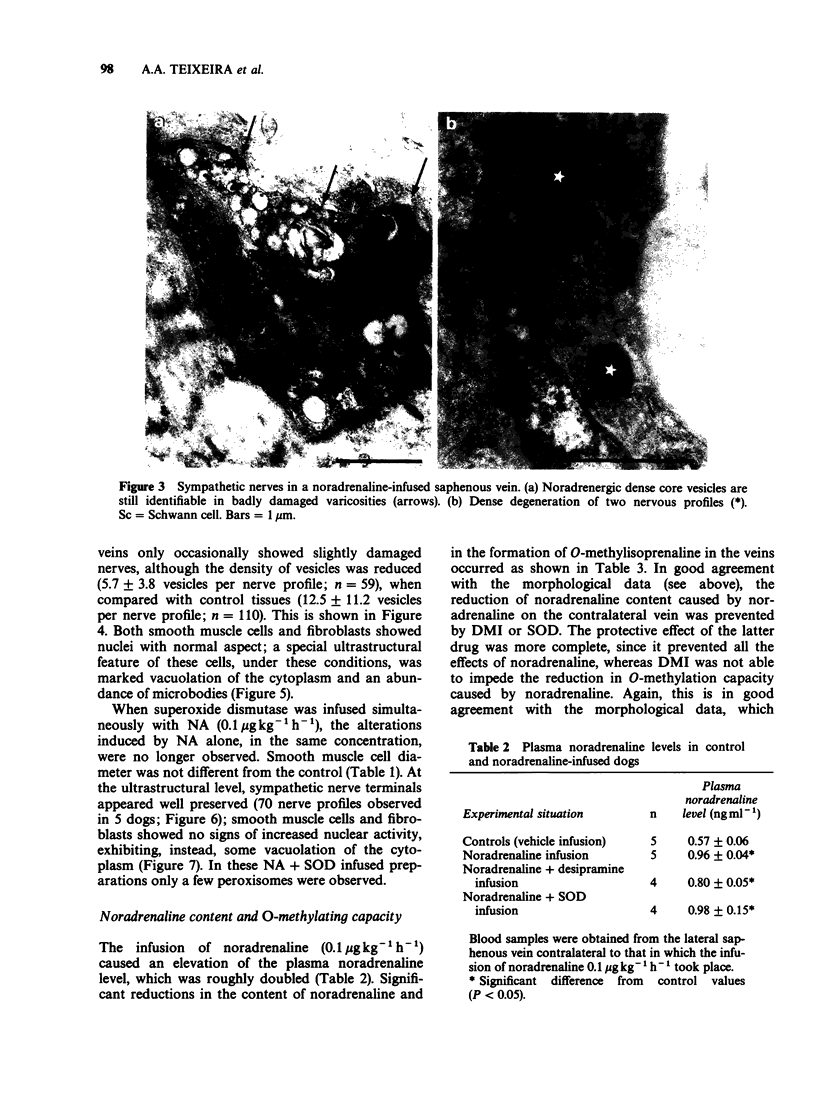
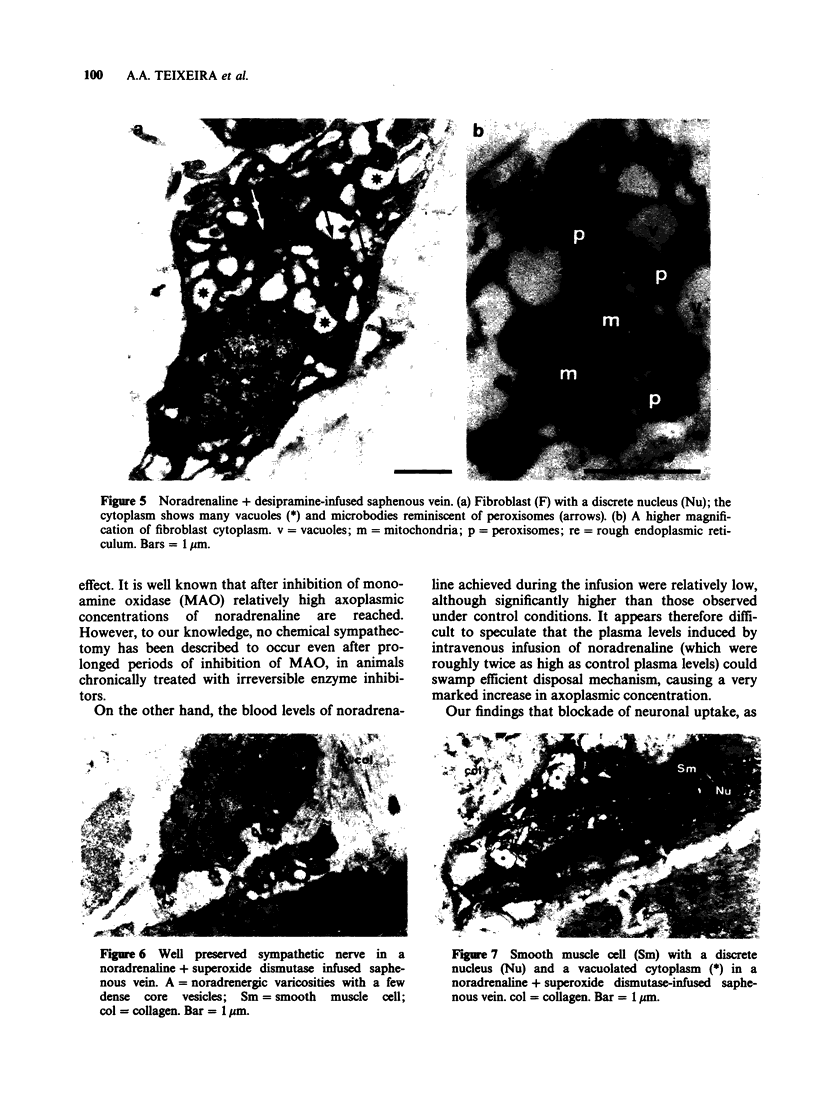
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azevedo I., Castro-Tavares J., Garrett J. Ultrastructural changes in blood vessels of perinephritic hypertensive dogs. Blood Vessels. 1981;18(3):110–119. doi: 10.1159/000158343. [DOI] [PubMed] [Google Scholar]
- Azevedo I., Osswald W. Adrenergic nerve degeneration induced by condensation products of adrenaline and acetaldehyde. Naunyn Schmiedebergs Arch Pharmacol. 1977 Nov;300(2):139–144. doi: 10.1007/BF00505044. [DOI] [PubMed] [Google Scholar]
- Azevedo I., Osswald W. Trophic role of the sympathetic innervation. J Pharmacol. 1986;17 (Suppl 2):30–43. [PubMed] [Google Scholar]
- Azevedo I., Osswald W. Uptake, distribution and metabolism of isoprenaline in the dog saphenous vein. Naunyn Schmiedebergs Arch Pharmacol. 1976 Nov;295(2):141–147. doi: 10.1007/BF00499446. [DOI] [PubMed] [Google Scholar]
- Branco D., Teixeira A. A., Azevedo I., Osswald W. Structural and functional alterations caused at the extraneuronal level by sympathetic denervation of blood vessels. Naunyn Schmiedebergs Arch Pharmacol. 1984 Jul;326(4):302–312. doi: 10.1007/BF00501434. [DOI] [PubMed] [Google Scholar]
- Coimbra A., Ribeiro-Silva A., Osswald W. Fine structural and autoradiographic study of the adrenergic innervation of the dog lateral saphenous vein. Blood Vessels. 1974;11(3):128–144. doi: 10.1159/000158007. [DOI] [PubMed] [Google Scholar]
- Collins M. A., Hannigan J. J., Origitano T., Moura D., Osswald W. On the occurrence, assay and metabolism of simple tetrahydroisoquinolines in mammalian tissues. Prog Clin Biol Res. 1982;90:155–166. [PubMed] [Google Scholar]
- Dimitriadou V., Aubineau P., Taxi J., Seylaz J. Ultrastructural changes in the cerebral artery wall induced by long-term sympathetic denervation. Blood Vessels. 1988;25(3):122–143. doi: 10.1159/000158727. [DOI] [PubMed] [Google Scholar]
- Graham D. G. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978 Jul;14(4):633–643. [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Marklund S. Distribution of CuZn superoxide dismutase and Mn superoxide dismutase in human tissues and extracellular fluids. Acta Physiol Scand Suppl. 1980;492:19–23. [PubMed] [Google Scholar]
- Osswald W., Azevedo I. Chemical sympathectomy due to tetrahydroisoquinolines derived from adrenaline. Adv Exp Med Biol. 1980;126:131–136. doi: 10.1007/978-1-4684-3632-7_12. [DOI] [PubMed] [Google Scholar]
- STONE C. A., PORTER C. C., STAVORSKI J. M., LUDDEN C. T., TOTARO J. A. ANTAGONISM OF CERTAIN EFFECTS OF CATECHOLAMINE-DEPLETING AGENTS BY ANTIDEPRESSANT AND RELATED DRUGS. J Pharmacol Exp Ther. 1964 May;144:196–204. [PubMed] [Google Scholar]
- Saner A., Thoenen H. Model experiments on the molecular mechanism of action of 6-hydroxydopamine. Mol Pharmacol. 1971 Mar;7(2):147–154. [PubMed] [Google Scholar]
- Sarmento A., Soares-da-Silva P., Teixeira A. A., Azevedo I. Effects of denervation induced by 6-hydroxydopamine on cell nucleus activity of arterial and cardiac cells of the dog. J Auton Pharmacol. 1987 Jun;7(2):119–126. doi: 10.1111/j.1474-8673.1987.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Van Vliet P. D., Burchell H. B., Titus J. L. Focal myocarditis associated with pheochromocytoma. N Engl J Med. 1966 May 19;274(20):1102–1108. doi: 10.1056/NEJM196605192742002. [DOI] [PubMed] [Google Scholar]