Abstract
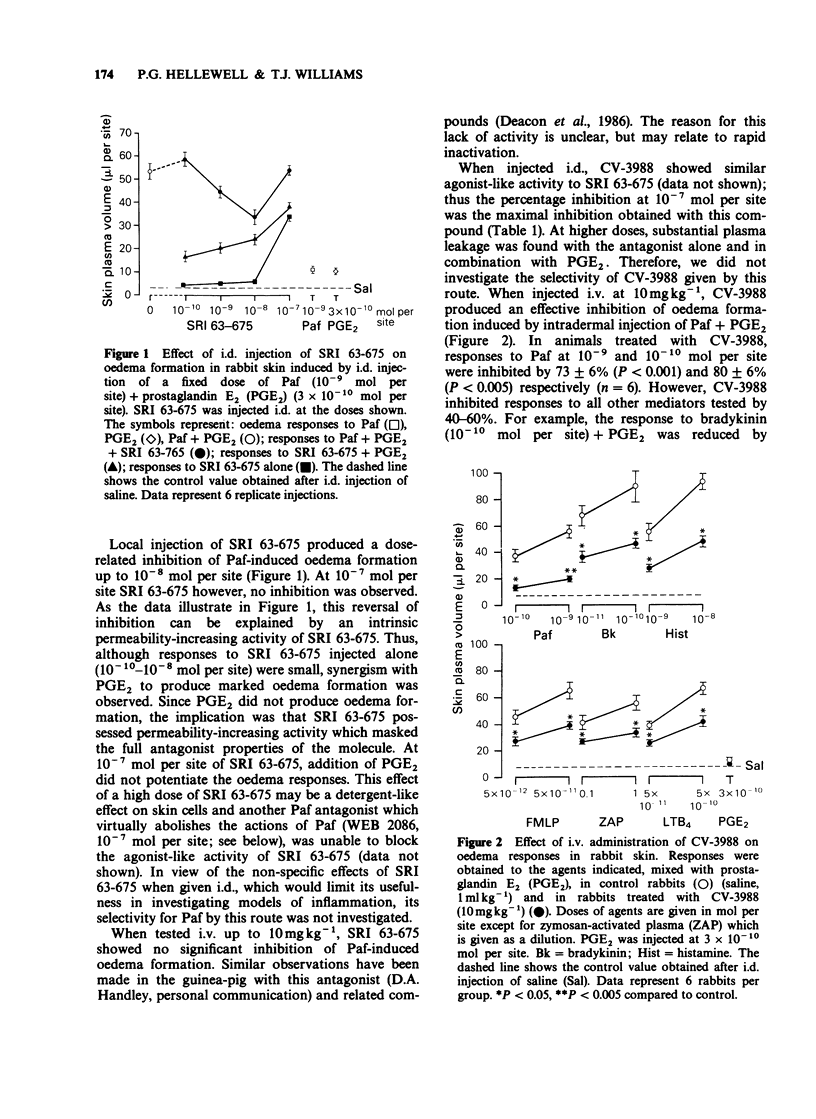
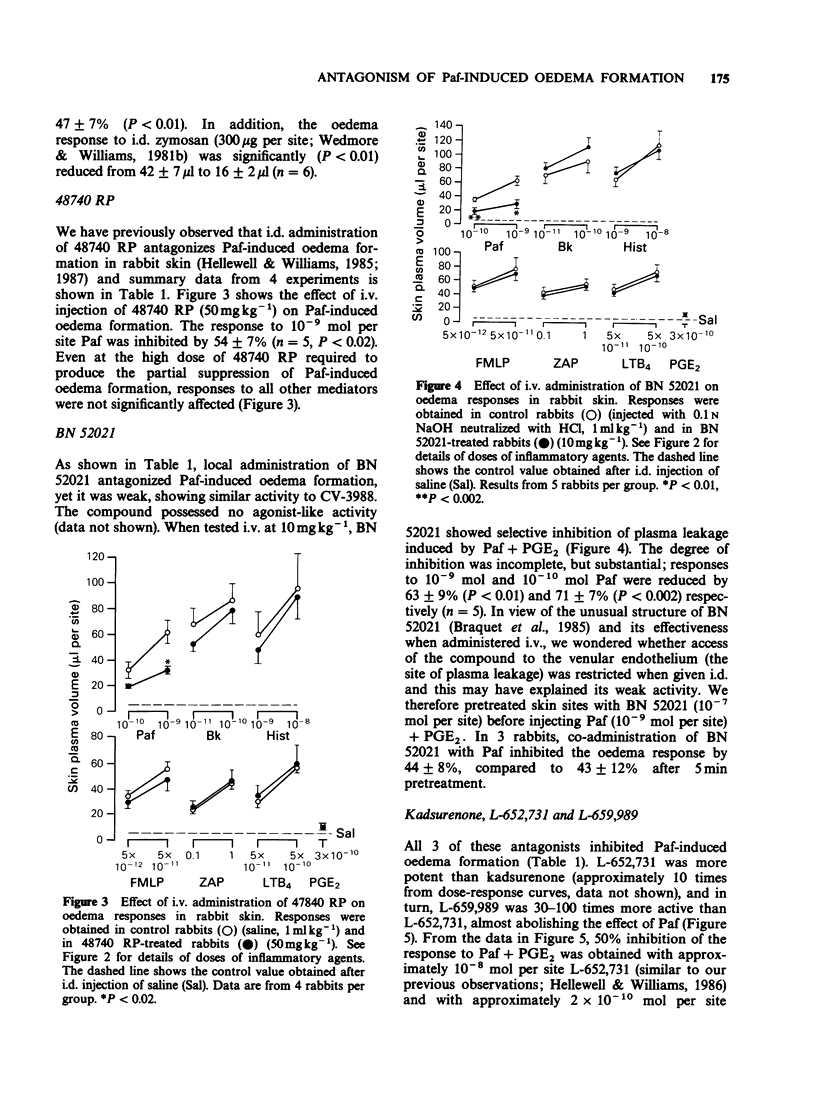
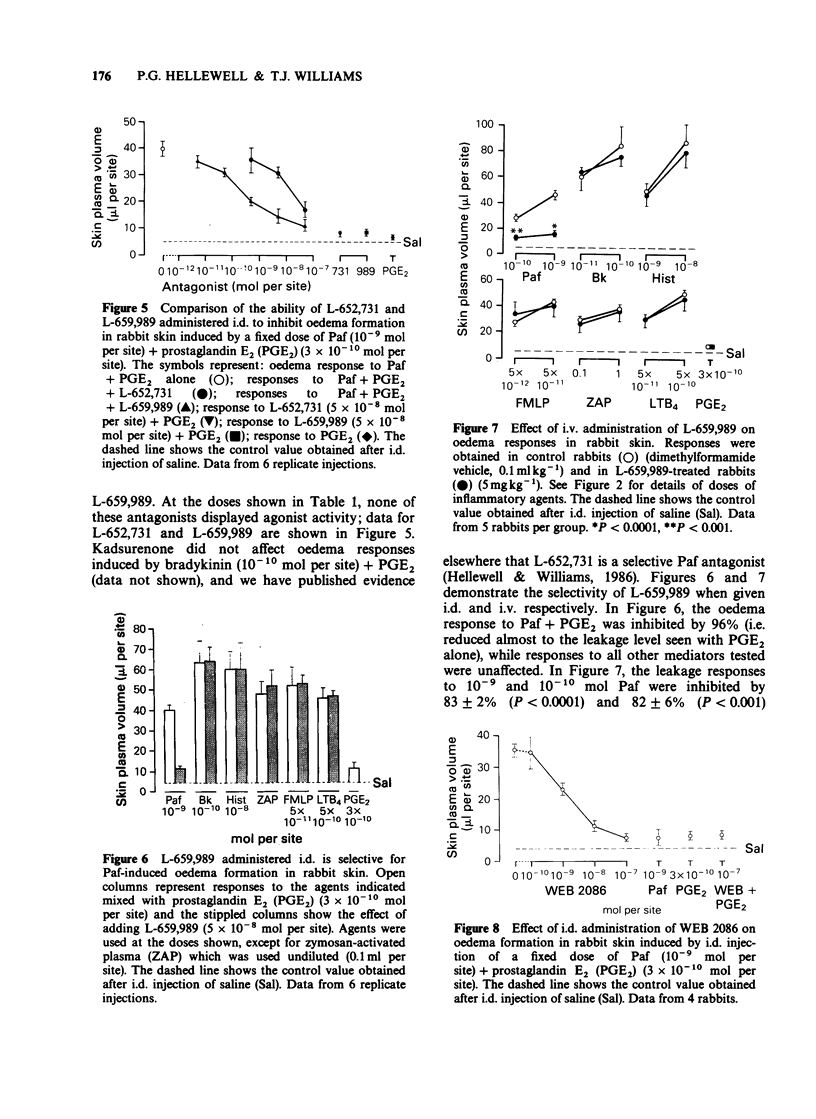
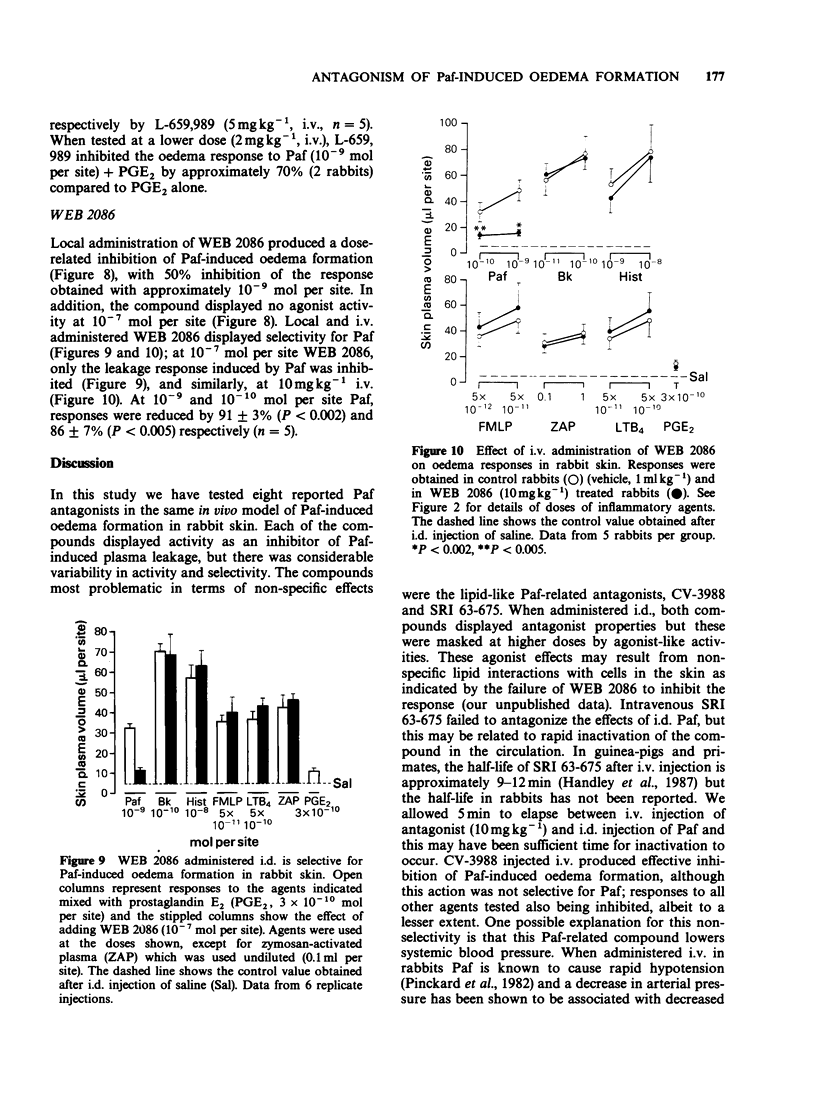
1. Eight platelet activating factor (Paf) antagonists were evaluated as inhibitors of oedema formation in rabbit skin induced by intradermal injection of Paf plus prostaglandin E2 (PGE2). Antagonists were tested by both intradermal (i.d.) and intravenous (i.v.) routes. 2. Intradermal injection of two antagonists structurally-related to Paf (SRI 63-675 and CV-3988) resulted in a partial inhibition of Paf-induced oedema formation but at high doses of antagonist, marked agonist activities were detected. CV-3988 administered i.v. inhibited Paf-induced plasma leakage by 73-80%; however, oedema responses to a range of other inflammatory mediators were also reduced, albeit to a lesser extent (40-60%). SRI 63-675 administered i.v. did not significantly inhibit Paf-induced oedema. 3. The antagonist 48740 RP administered either i.d. or i.v. showed partial, but selective, inhibition of Paf-induced oedema formation, although the doses required were high when compared with other antagonists. 4. BN 52021 was a weak Paf antagonist when injected i.d., but following i.v. administration the responses to Paf were inhibited by 63-71%. Responses to all other mediators tested were unaffected. 5. Kadsurenone and its synthetic derivatives, L-652,731 and L-659,989 all blocked responses to Paf in the skin. L-659,989 was the most potent, achieving almost total inhibition when injected i.d. and i.v.; moreover, it was selective for Paf. L-652,731 was more potent than kadsurenone. 6. WEB 2086 given i.d. and i.v. showed similar activity to L-659,989 and it was also selective for Paf-induced oedema formation. 7. These results illustrate that in rabbit skin not all Paf antagonists are selective for Paf, some showing agonist-like activity which can mask antagonist properties. It is suggested that before ascribing a role for endogenous Paf in an inflammatory reaction based on results with antagonists, the activity of the antagonists in the model under investigation should be rigorously established.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braquet P., Etienne A., Touvay C., Bourgain R. H., Lefort J., Vargaftig B. B. Involvement of platelet activating factor in respiratory anaphylaxis, demonstrated by PAF-acether inhibitor BN 52021. Lancet. 1985 Jun 29;1(8444):1501–1501. doi: 10.1016/s0140-6736(85)92269-x. [DOI] [PubMed] [Google Scholar]
- Braquet P., Touqui L., Shen T. Y., Vargaftig B. B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987 Jun;39(2):97–145. [PubMed] [Google Scholar]
- Casals-Stenzel J., Muacevic G., Weber K. H. Pharmacological actions of WEB 2086, a new specific antagonist of platelet activating factor. J Pharmacol Exp Ther. 1987 Jun;241(3):974–981. [PubMed] [Google Scholar]
- Chung K. F., Dent G., McCusker M., Guinot P., Page C. P., Barnes P. J. Effect of a ginkgolide mixture (BN 52063) in antagonising skin and platelet responses to platelet activating factor in man. Lancet. 1987 Jan 31;1(8527):248–251. doi: 10.1016/s0140-6736(87)90066-3. [DOI] [PubMed] [Google Scholar]
- Evans T. W., Dent G., Rogers D. F., Aursudkij B., Chung K. F., Barnes P. J. Effect of a Paf antagonist, WEB 2086, on airway microvascular leakage in the guinea-pig and platelet aggregation in man. Br J Pharmacol. 1988 May;94(1):164–168. doi: 10.1111/j.1476-5381.1988.tb11511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley D. A., Van Valen R. G., Winslow C. M., Tomesch J. C., Saunders R. N. In vitro and in vivo pharmacological profiles of the PAF receptor antagonist SRI 63-675. Thromb Haemost. 1987 Apr 7;57(2):187–190. [PubMed] [Google Scholar]
- Hellewell P. G., Williams T. J. A specific antagonist of platelet-activating factor suppresses oedema formation in an Arthus reaction but not oedema induced by leukocyte chemoattractants in rabbit skin. J Immunol. 1986 Jul 1;137(1):302–307. [PubMed] [Google Scholar]
- Humphrey D. M., McManus L. M., Satouchi K., Hanahan D. J., Pinckard R. N. Vasoactive properties of acetyl glyceryl ether phosphorylcholine and analogues. Lab Invest. 1982 Apr;46(4):422–427. [PubMed] [Google Scholar]
- Issekutz A. C., Szpejda M. Evidence that platelet activating factor may mediate some acute inflammatory responses. Studies with the platelet-activating factor antagonist, CV3988. Lab Invest. 1986 Mar;54(3):275–281. [PubMed] [Google Scholar]
- Jose P. J., Forrest M. J., Williams T. J. Detection of the complement fragment C5a in inflammatory exudates from the rabbit peritoneal cavity using radioimmunoassay. J Exp Med. 1983 Dec 1;158(6):2177–2182. doi: 10.1084/jem.158.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivern D. V., Basran G. S. Synergism between platelet-activating factor (PAF-acether) and prostaglandin E2 in man. Eur J Pharmacol. 1984 Jun 15;102(1):183–185. doi: 10.1016/0014-2999(84)90356-x. [DOI] [PubMed] [Google Scholar]
- Morley J., Page C. P., Paul W. Inflammatory actions of platelet activating factor (Pafacether) in guinea-pig skin. Br J Pharmacol. 1983 Nov;80(3):503–509. doi: 10.1111/j.1476-5381.1983.tb10722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotzky E., Page C. P., Roubin R., Pfister A., Paul W., Bonnet J., Benveniste J. PAF-acether-induced plasma exudation in rat skin is independent of platelets and neutrophils. Microcirc Endothelium Lymphatics. 1984 Feb;1(1):107–122. [PubMed] [Google Scholar]
- Ponpipom M. M., Hwang S. B., Doebber T. W., Acton J. J., Alberts A. W., Biftu T., Brooker D. R., Bugianesi R. L., Chabala J. C., Gamble N. L. (+/-)-trans-2-(3-Methoxy-5-methylsulfonyl-4-propoxyphenyl)-5-(3,4,5- trimethoxyphenyl)tetrahydrofuran (L-659,989), a novel, potent PAF receptor antagonist. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1213–1220. doi: 10.1016/0006-291x(88)90758-9. [DOI] [PubMed] [Google Scholar]
- Rampart M., Williams T. J. Polymorphonuclear leukocyte-dependent plasma leakage in the rabbit skin is enhanced or inhibited by prostacyclin, depending on the route of administration. Am J Pathol. 1986 Jul;124(1):66–73. [PMC free article] [PubMed] [Google Scholar]
- Saunders R. N., Handley D. A. Platelet-activating factor antagonists. Annu Rev Pharmacol Toxicol. 1987;27:237–255. doi: 10.1146/annurev.pa.27.040187.001321. [DOI] [PubMed] [Google Scholar]
- Shen T. Y., Hwang S. B., Chang M. N., Doebber T. W., Lam M. H., Wu M. S., Wang X., Han G. Q., Li R. Z. Characterization of a platelet-activating factor receptor antagonist isolated from haifenteng (Piper futokadsura): specific inhibition of in vitro and in vivo platelet-activating factor-induced effects. Proc Natl Acad Sci U S A. 1985 Feb;82(3):672–676. doi: 10.1073/pnas.82.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashita Z., Tsushima S., Yoshioka Y., Nomura H., Inada Y., Nishikawa K. CV-3988 - a specific antagonist of platelet activating factor (PAF). Life Sci. 1983 Apr 25;32(17):1975–1982. doi: 10.1016/0024-3205(83)90049-8. [DOI] [PubMed] [Google Scholar]
- Voelkel N. F., Chang S. W., Pfeffer K. D., Worthen S. G., McMurtry I. F., Henson P. M. PAF antagonists: different effects on platelets, neutrophils, guinea pig ileum and PAF-induced vasodilation in isolated rat lung. Prostaglandins. 1986 Sep;32(3):359–372. doi: 10.1016/0090-6980(86)90005-5. [DOI] [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]


