Abstract
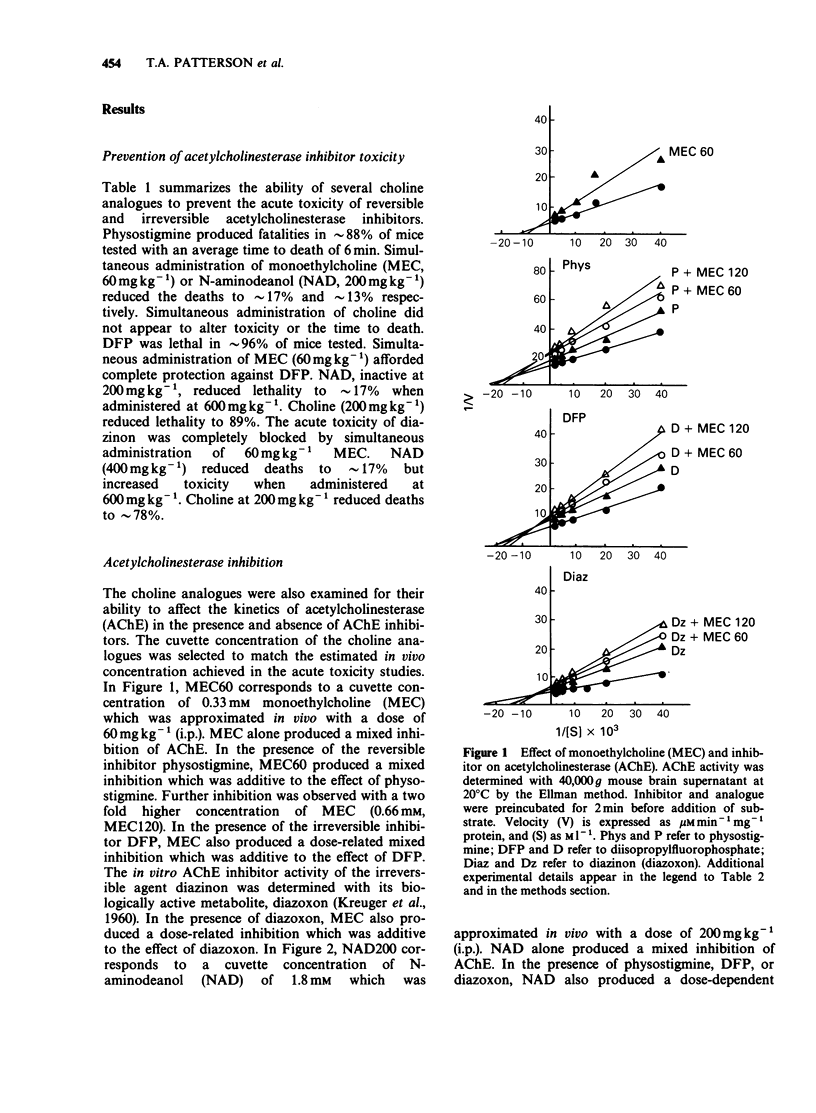
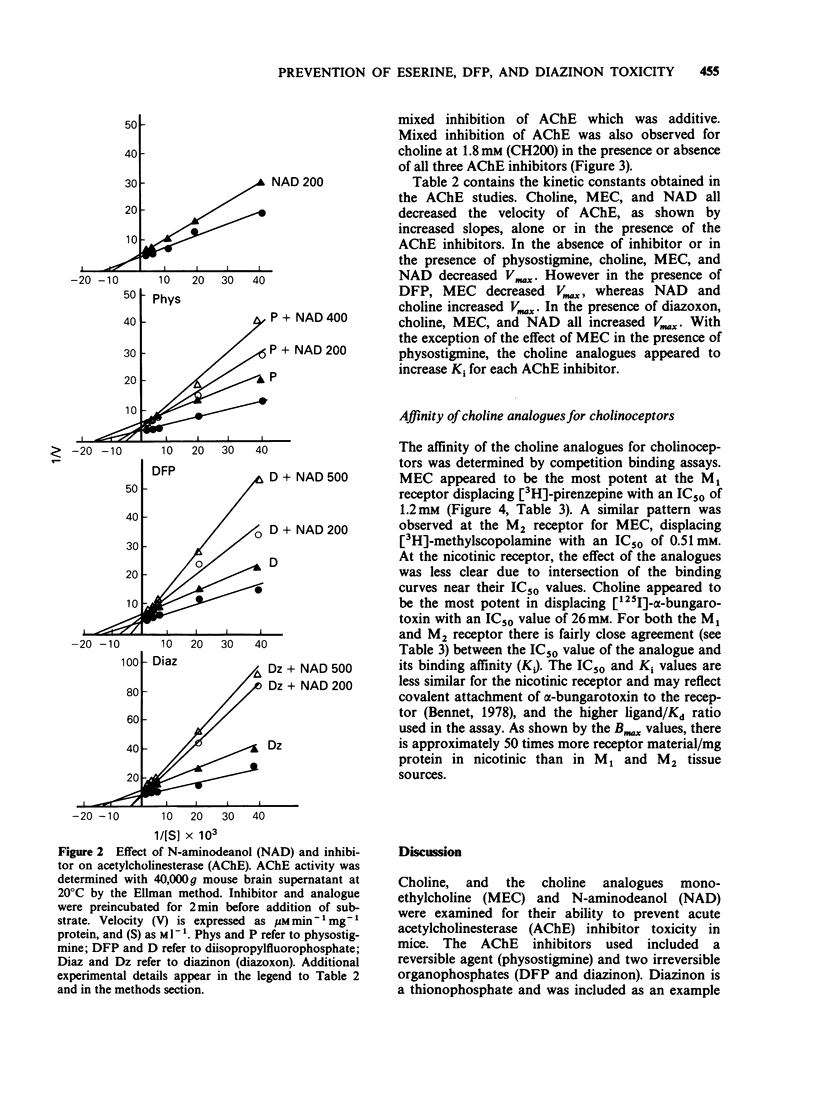
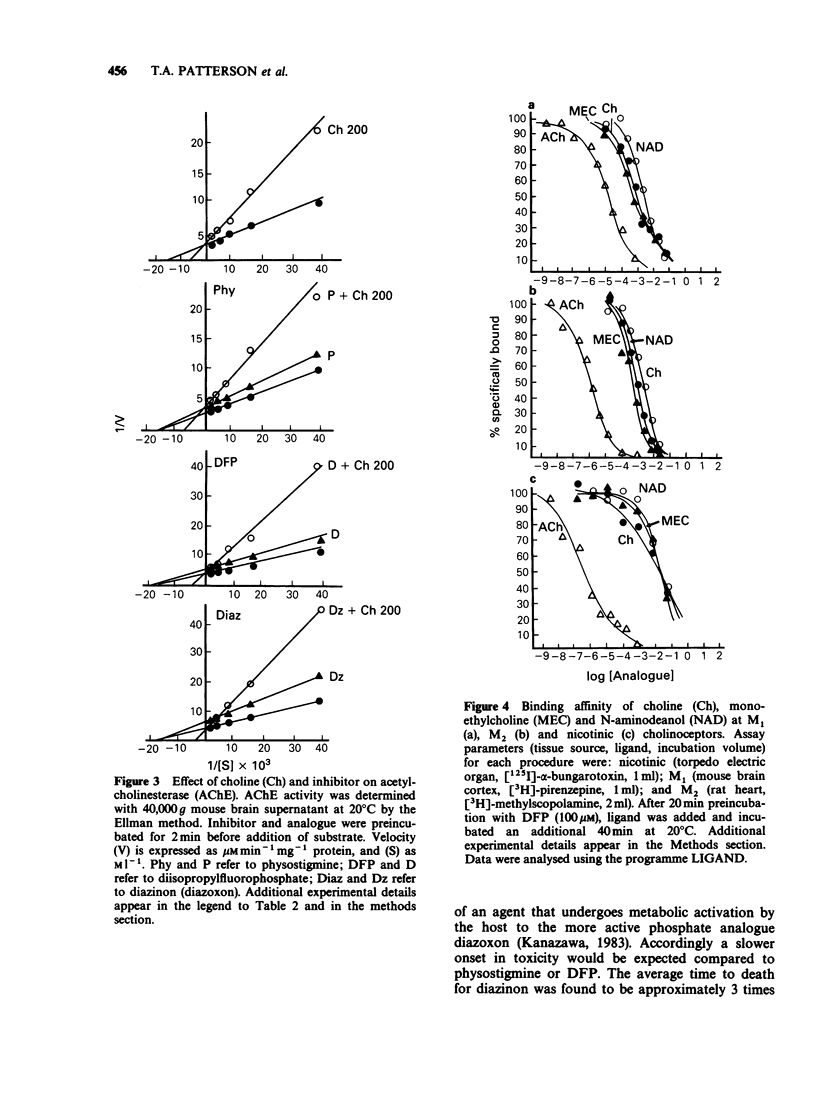
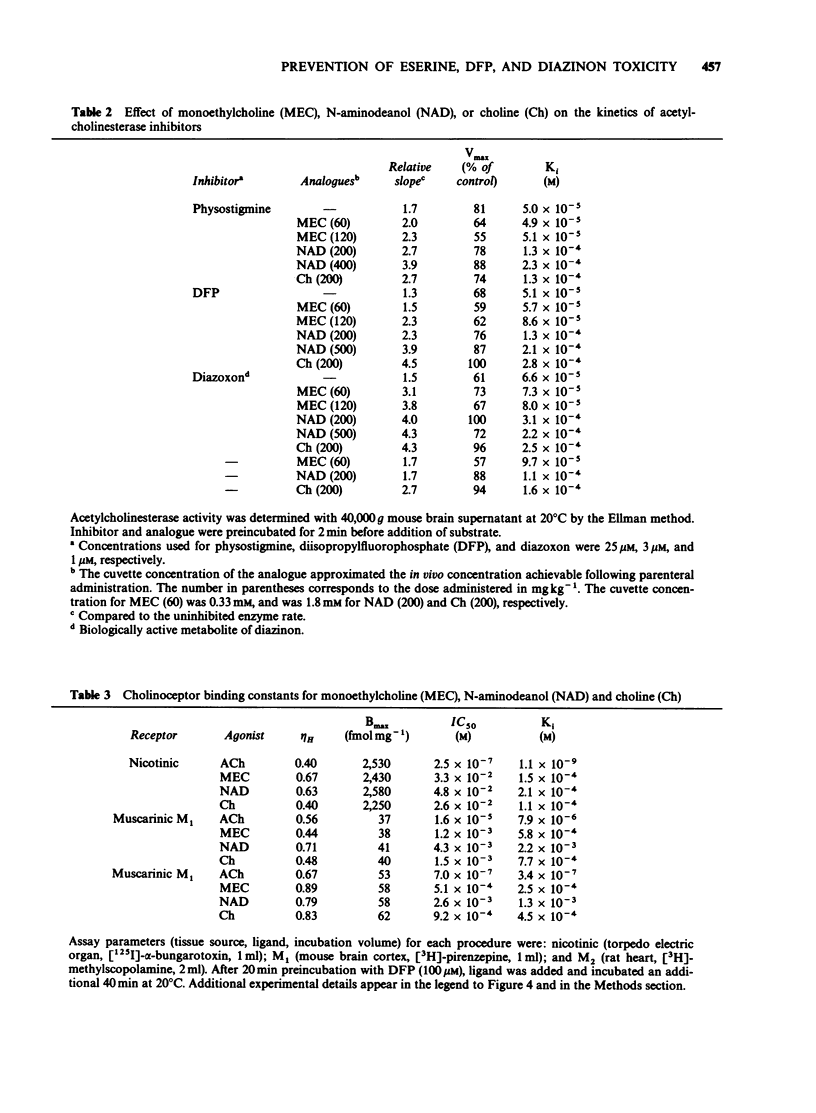
1. Choline, and the choline analogues monoethylcholine (MEC) and N-aminodeanol (NAD) were examined for prophylactic activity in acute acetylcholinesterase inhibitor toxicity in mice. The rank order of potency of the compounds was MEC greater than NAD greater than choline. 2. Simultaneous administration of MEC (60 mg kg-1) or NAD (200 mg kg-1) with physostigmine reduced lethality to 17 and 13% respectively. MEC (60 mg kg-1) completely protected against disopropylfluorophosphate (DFP) and diazinon toxicity, and NAD reduced lethality to 17% for both agents. Choline (200 mg kg-1) exhibited only negligible antidotal activity against the inhibitors. 3. In vitro concentrations of choline, MEC, and NAD, similar to the estimated concentration obtained in vivo in the acute toxicity study, produced mixed inhibition of mouse brain acetylcholinesterase. The inhibition was dose-related and was additive to the inhibition produced by the cholinesterase inhibitors. 4. All three analogues reduced ligand binding at the nicotinic, M1, and M2 receptors. The rank order of potencies for the analogues at each receptor was nicotinic: (choline greater than MEC greater than NAD), M1: (MEC greater than choline greater than NAD), and M2: (MEC greater than choline greater than NAD). 5. It is proposed that the analogues prevent acetylcholinesterase inhibitor toxicity peripherally by interacting with acetylcholinesterase, and/or by competing with acetylcholine for binding to cholinoceptors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronstam R. S., Abood L. G., Hoss W. Influence of sulfhydryl reagents and heavy metals on the functional state of the muscarinic acetylcholine receptor in rat brain. Mol Pharmacol. 1978 Jul;14(4):575–586. [PubMed] [Google Scholar]
- Aronstam R. S., Smith M. D., Buccafusco J. J. Clonidine protection from soman and echothiophate toxicity in mice. Life Sci. 1986 Dec 1;39(22):2097–2102. doi: 10.1016/0024-3205(86)90361-9. [DOI] [PubMed] [Google Scholar]
- BARTLETT J. P., ADAMS W. E. Generalized giant hypertrophic gastritis simulating neoplasm; differential diagnosis and report of a case. Arch Surg. 1950 Mar;60(3):543–558. doi: 10.1001/archsurg.1950.01250010562011. [DOI] [PubMed] [Google Scholar]
- Buccafusco J. J., Aronstam R. S. Precursors of cholinergic false transmitters: central effects on blood pressure and direct interactions with cholinergic receptors. Neuropharmacology. 1988 Mar;27(3):227–233. doi: 10.1016/0028-3908(88)90038-x. [DOI] [PubMed] [Google Scholar]
- Chemnitius J. M., Haselmeyer K. H., Zech R. Brain cholinesterases. Differentiation of target enzymes for toxic organophosphorus compounds. Biochem Pharmacol. 1983 Jun 1;32(11):1693–1699. doi: 10.1016/0006-2952(83)90111-9. [DOI] [PubMed] [Google Scholar]
- Clement J. G., Lockwood P. A. HI-6, an oxime which is an effective antidote of soman poisoning: a structure-activity study. Toxicol Appl Pharmacol. 1982 Jun 15;64(1):140–146. doi: 10.1016/0041-008x(82)90332-5. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fleisher J. H., Harris L. W. Dealkylation as a mechanism for aging of cholinesterase after poisoning with pinacolyl methylphosphonofluoridate. Biochem Pharmacol. 1965 May;14(5):641–650. doi: 10.1016/0006-2952(65)90082-1. [DOI] [PubMed] [Google Scholar]
- HOLMES R. Discussion on poisoning with anticholinesterases. Proc R Soc Med. 1953 Oct;46(10):799–800. [PMC free article] [PubMed] [Google Scholar]
- Harris L. W., Heyl W. C., Stitcher D. L., Moore R. D. Effect of atropine and/or physostigmine on cerebral acetylcholine in rats poisoned with soman. Life Sci. 1978 Mar;22(10):907–910. doi: 10.1016/0024-3205(78)90615-x. [DOI] [PubMed] [Google Scholar]
- Leadbeater L., Inns R. H., Rylands J. M. Treatment of poisoning by soman. Fundam Appl Toxicol. 1985 Dec;5(6 Pt 2):S225–S231. doi: 10.1016/0272-0590(85)90132-0. [DOI] [PubMed] [Google Scholar]
- Lin J., Freeman J. J., Kosh J. W. Antagonism of acute physostigmine and neostigmine toxicity in mice by hemicholinium-3. Res Commun Chem Pathol Pharmacol. 1987 Apr;56(1):137–140. [PubMed] [Google Scholar]
- Meeter E., Wolthuis O. L. The spontaneous recovery of respiration and neuromuscular transmission in the rat after anticholinesterase poisoning. Eur J Pharmacol. 1968 Mar;2(5):377–386. doi: 10.1016/0014-2999(68)90189-1. [DOI] [PubMed] [Google Scholar]
- Natoff I. L., Reiff B. Effect of oximes on the acute toxicity of anticholinesterase carbamates. Toxicol Appl Pharmacol. 1973 Aug;25(4):569–575. doi: 10.1016/0041-008x(73)90026-4. [DOI] [PubMed] [Google Scholar]
- Newton M. W., Ringdahl B., Jenden D. J. Estimation of N-amino-N,N-dimethylaminoethanol, choline and their acetate esters by gas chromatography mass spectrometry. Anal Biochem. 1983 Apr 1;130(1):88–94. doi: 10.1016/0003-2697(83)90653-x. [DOI] [PubMed] [Google Scholar]
- Okonek S., Kilbinger H. Determination of acetylcholine, nitrostigmine and acetylcholinesterase activity in four patients with severe nitrostigmine (E 605 forte) intoxication. Arch Toxicol. 1974;32(2):97–108. doi: 10.1007/BF00316230. [DOI] [PubMed] [Google Scholar]
- STOVNER J. The effect of choline on the action of anticholinesterases. Acta Pharmacol Toxicol (Copenh) 1956;12(2):175–186. doi: 10.1111/j.1600-0773.1956.tb01374.x. [DOI] [PubMed] [Google Scholar]
- Schoene K., Steinhanses J., Oldiges H. Protection against organophosphate poisoning in vivo and inhibition of choline-acetyltransferase in vitro. Biochem Pharmacol. 1977 Oct 1;26(19):1821–1823. doi: 10.1016/0006-2952(77)90354-9. [DOI] [PubMed] [Google Scholar]
- Sellers D. J., Watts P., Wilkinson R. G. Interactions between acetylcholinesterase and tetra-N-alkylammonium ions. Biochem Pharmacol. 1983 Mar 1;32(5):787–793. doi: 10.1016/0006-2952(83)90577-4. [DOI] [PubMed] [Google Scholar]
- Watts P., Wilkinson R. G. The interaction of carbamates with acetylcholinesterase. Biochem Pharmacol. 1977 Apr 15;26(8):757–761. doi: 10.1016/0006-2952(77)90220-9. [DOI] [PubMed] [Google Scholar]
- Wolthuis O. L., Benschop H. P., Berends F. Persistence of the anti-cholinesterase soman in rats; antagonism with a non-toxic simulator of this organophosphate. Eur J Pharmacol. 1981 Jan 29;69(3):379–383. doi: 10.1016/0014-2999(81)90488-x. [DOI] [PubMed] [Google Scholar]
- van Helden H. P., van der Wiel H. J., Wolthuis O. L. Therapy of organophosphate poisoning: the marmoset as a model for man. Br J Pharmacol. 1983 Mar;78(3):579–589. doi: 10.1111/j.1476-5381.1983.tb08818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


