Abstract
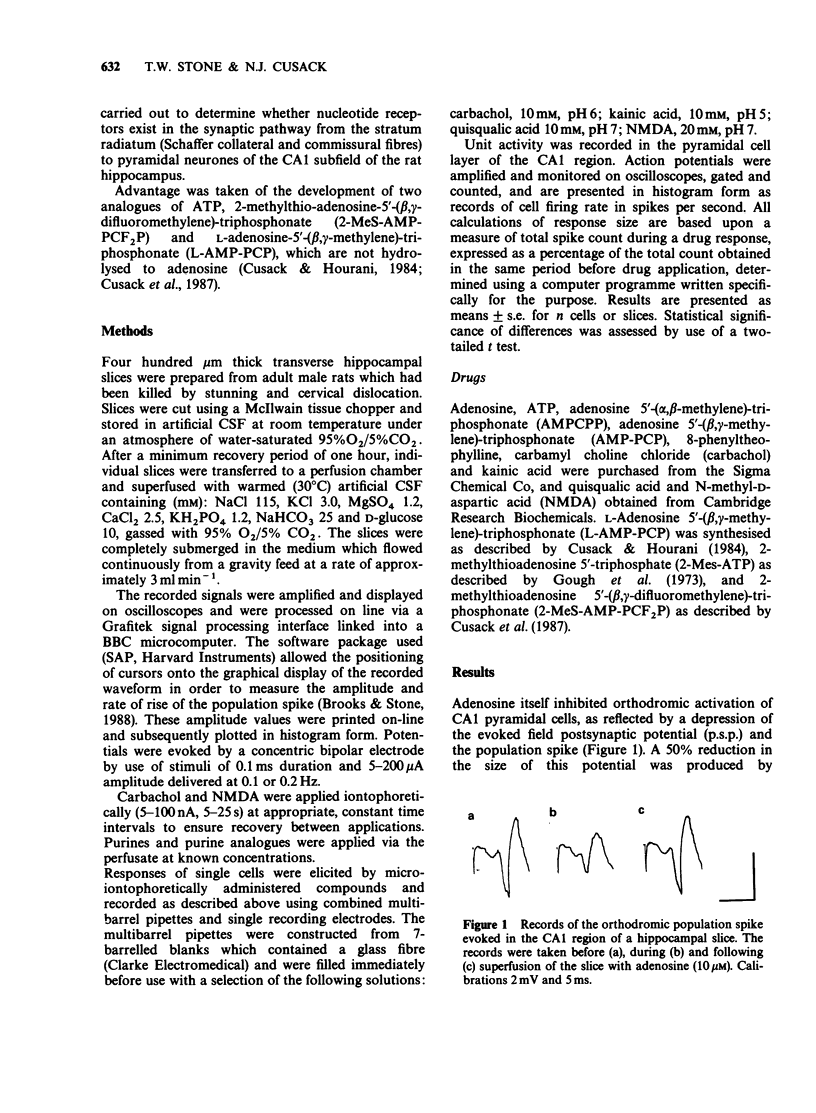
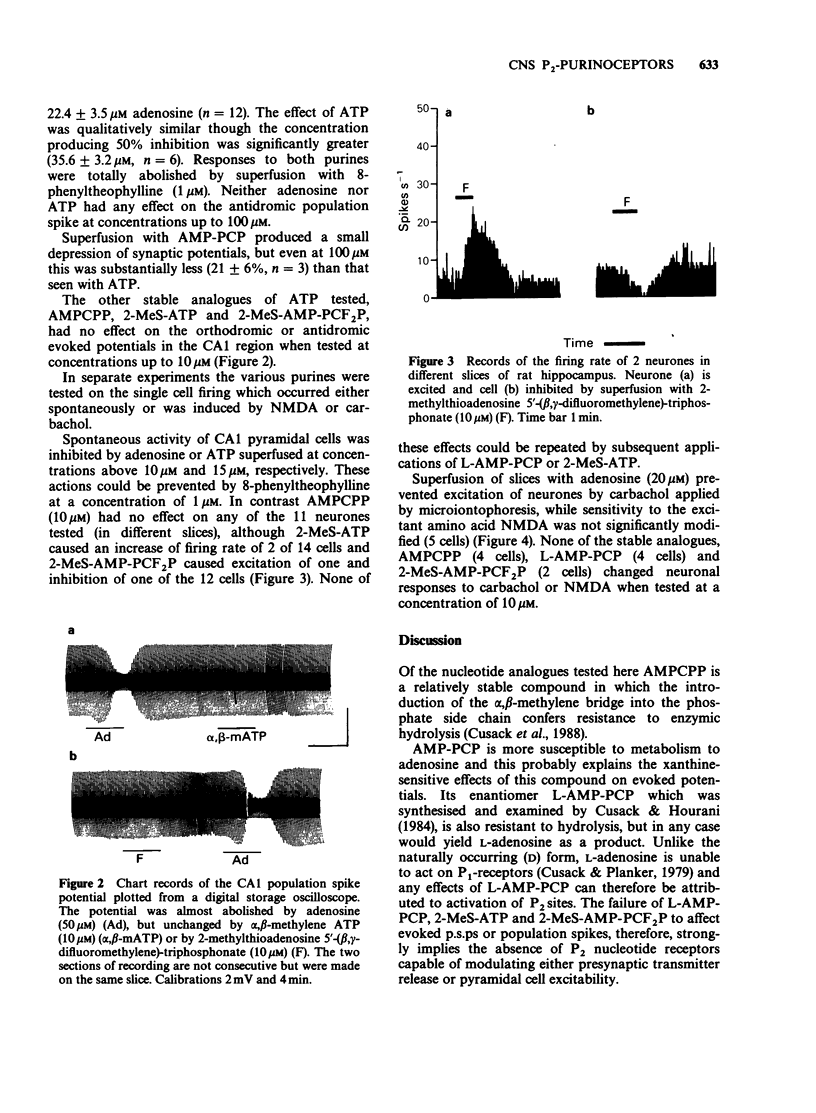
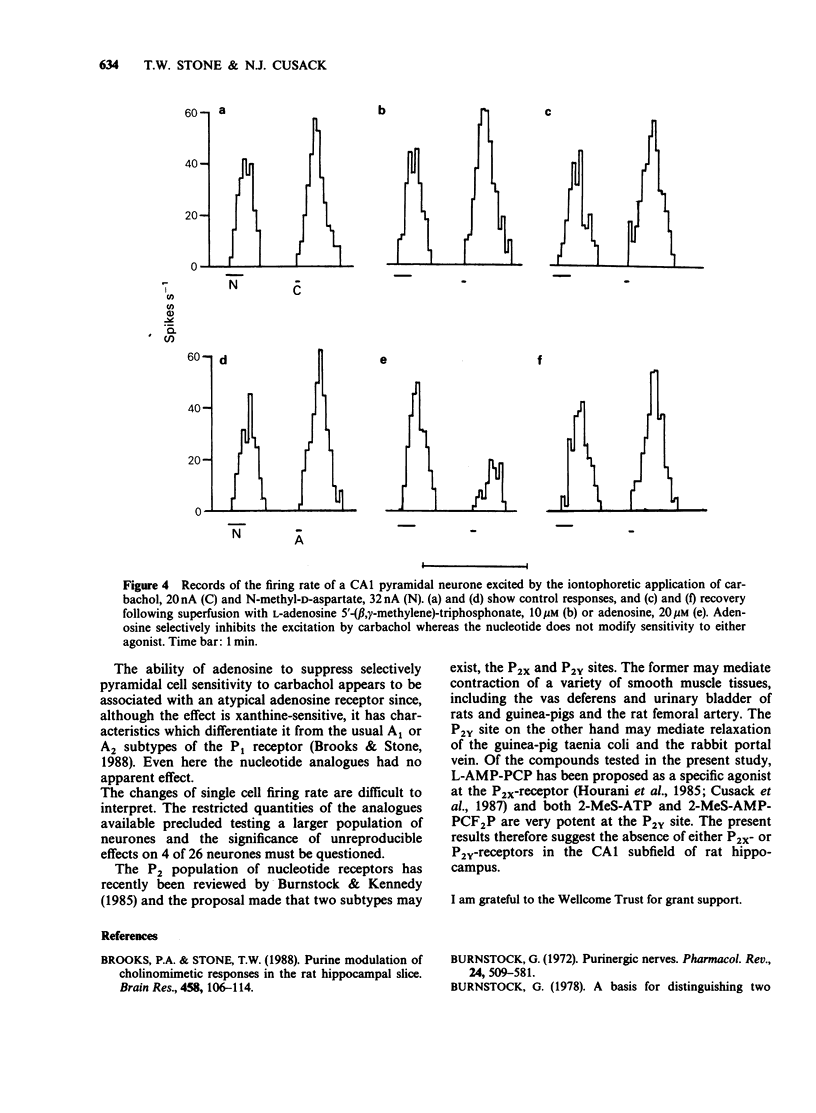
1. Many apparent actions of adenosine 5'-triphosphate (ATP) are mediated by adenosine produced by enzymatic hydrolysis of the nucleotide. Previously described actions of ATP in the CNS have been partly due to this phenomenon. In the present study analogues of ATP, which are not hydrolysed to adenosine, were used to seek responses to activating nucleotide (P2) receptors in the hippocampus. The analogues used were L-adenosine-5'-(beta,gamma-methylene)-triphosphonate and 2-methylthioadenosine-5'-(beta,gamma-difluoromethylene)-triphosphonat e. 2. Neither of the stable nucleotides had any effect on orthodromically evoked synaptic potentials in the CA1 region of rat hippocampal slices. Adenosine and ATP had inhibitory actions that could be prevented by the P1-receptor blocker 8-phenyltheophylline. 3. The stable nucleotides had no consistent effects on the firing rate of single neurones in stratum pyramidale of the CA1 region, although adenosine and ATP produced a xanthine-sensitive inhibition. 4. Adenosine selectively reduced the sensitivity of CA1 neurones to microiontophoretically applied carbachol whereas stable nucleotides did not. 5. It is concluded that there are neither P2x- nor P2y-receptors for adenine nucleotides on rat hippocampal CA1 pyramidal cells at the Schaffer collateral and commissural terminals in stratum radiatum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks P. A., Stone T. W. Purine modulation of cholinomimetic responses in the rat hippocampal slice. Brain Res. 1988 Aug 16;458(1):106–114. doi: 10.1016/0006-8993(88)90501-x. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Cusack N. J., Hourani S. M., Loizou G. D., Welford L. A. Pharmacological effects of isopolar phosphonate analogues of ATP on P2-purinoceptors in guinea-pig taenia coli and urinary bladder. Br J Pharmacol. 1987 Apr;90(4):791–795. doi: 10.1111/j.1476-5381.1987.tb11233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack N. J., Hourani S. M. Some pharmacological and biochemical interactions of the enantiomers of adenylyl 5'-(beta, gamma-methylene)-diphosphonate with the guinea-pig urinary bladder. Br J Pharmacol. 1984 May;82(1):155–159. doi: 10.1111/j.1476-5381.1984.tb16453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack N. J., Planker M. Relaxation of isolated taenia coli of guinea-pig by enantiomers of 2-azido analogues of adenosine and adenine nucleotides. Br J Pharmacol. 1979 Sep;67(1):153–158. [PMC free article] [PubMed] [Google Scholar]
- Fedan J. S., Hogaboom G. K., O'Donnell J. P., Colby J., Westfall D. P. Contribution by purines to the neurogenic response of the vas deferens of the guinea pig. Eur J Pharmacol. 1981 Jan 5;69(1):41–53. doi: 10.1016/0014-2999(81)90600-2. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Hedqvist P. Modulation of neurotransmission by purine nucleotides and nucleosides. Biochem Pharmacol. 1980 Jun 15;29(12):1635–1643. doi: 10.1016/0006-2952(80)90117-3. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough G. R., Maguire M. H., Satchell D. G. Three new adenosine triphosphate analogs. Synthesis and effects on isolated gut. J Med Chem. 1973 Oct;16(10):1188–1190. doi: 10.1021/jm00268a028. [DOI] [PubMed] [Google Scholar]
- Hourani S. M., Welford L. A., Cusack N. J. L-AMP-PCP, an ATP receptor agonist in guinea-pig bladder, is inactive on taenia coli. Eur J Pharmacol. 1985 Jan 22;108(2):197–200. doi: 10.1016/0014-2999(85)90726-5. [DOI] [PubMed] [Google Scholar]
- Israël M., Lesbats B., Manaranche R., Meunier F. M., Frachon P. Retrograde inhibition of transmitter release by ATP. J Neurochem. 1980 Apr;34(4):923–932. doi: 10.1111/j.1471-4159.1980.tb09667.x. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Jessell T. M. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983 Aug 25;304(5928):730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- Machaly M., Dalziel H. H., Sneddon P. Evidence for ATP as a cotransmitter in dog mesenteric artery. Eur J Pharmacol. 1988 Feb 16;147(1):83–91. doi: 10.1016/0014-2999(88)90636-x. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H. The role of adenosine and its nucleotides in central synaptic transmission. Prog Neurobiol. 1981;16(3-4):187–239. doi: 10.1016/0301-0082(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. A. Purinergic modulation of transmitter release. J Theor Biol. 1979 Sep 21;80(2):259–270. doi: 10.1016/0022-5193(79)90210-8. [DOI] [PubMed] [Google Scholar]
- Salt T. E., Hill R. G. Excitation of single sensory neurones in the rat caudal trigeminal nucleus by iontophoretically applied adenosine 5'-triphosphate. Neurosci Lett. 1983 Jan 31;35(1):53–57. doi: 10.1016/0304-3940(83)90526-8. [DOI] [PubMed] [Google Scholar]
- Salter M. W., Henry J. L. Effects of adenosine 5'-monophosphate and adenosine 5'-triphosphate on functionally identified units in the cat spinal dorsal horn. Evidence for a differential effect of adenosine 5'-triphosphate on nociceptive vs non-nociceptive units. Neuroscience. 1985 Jul;15(3):815–825. doi: 10.1016/0306-4522(85)90080-6. [DOI] [PubMed] [Google Scholar]
- Schubert P., Heinemann U., Kolb R. Differential effect of adenosine on pre- and postsynaptic calcium fluxes. Brain Res. 1986 Jun 25;376(2):382–386. doi: 10.1016/0006-8993(86)90204-0. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984 Oct 30;106(1):149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984 Apr 13;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Fedan J. S. Cotransmitters in the motor nerves of the guinea pig vas deferens: electrophysiological evidence. Science. 1982 Nov 12;218(4573):693–695. doi: 10.1126/science.6291151. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Astrand P. Relative pre- and postjunctional roles of noradrenaline and adenosine 5'-triphosphate as neurotransmitters of the sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience. 1985 Mar;14(3):929–946. doi: 10.1016/0306-4522(85)90155-1. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Actions of adenine dinucleotides on the vas deferens, guinea-pig taenia caeci and bladder. Eur J Pharmacol. 1981 Oct 22;75(2-3):93–102. doi: 10.1016/0014-2999(81)90066-2. [DOI] [PubMed] [Google Scholar]
- Stone T. W., Perkins M. N. Adenine dinucleotide effects on rat cortical neurones. Brain Res. 1981 Dec 14;229(1):241–245. doi: 10.1016/0006-8993(81)90764-2. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Physiological roles for adenosine and adenosine 5'-triphosphate in the nervous system. Neuroscience. 1981;6(4):523–555. doi: 10.1016/0306-4522(81)90145-7. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Purine receptors involved in the depression of neuronal firing in cerebral cortex. Brain Res. 1982 Sep 30;248(2):367–370. doi: 10.1016/0006-8993(82)90596-0. [DOI] [PubMed] [Google Scholar]
- Su C. Purinergic neurotransmission and neuromodulation. Annu Rev Pharmacol Toxicol. 1983;23:397–411. doi: 10.1146/annurev.pa.23.040183.002145. [DOI] [PubMed] [Google Scholar]
- Westfall D. P., Fedan J. S., Colby J., Hogaboom G. K., O'Donnell J. P. Evidence for a contribution by purines to the neurogenic response of the guinea-pig urinary bladder. Eur J Pharmacol. 1983 Mar 4;87(4):415–422. doi: 10.1016/0014-2999(83)90080-8. [DOI] [PubMed] [Google Scholar]
- Wiklund N. P., Gustafsson L. E., Lundin J. Pre- and postjunctional modulation of cholinergic neuroeffector transmission by adenine nucleotides. Experiments with agonist and antagonist. Acta Physiol Scand. 1985 Dec;125(4):681–691. doi: 10.1111/j.1748-1716.1985.tb07771.x. [DOI] [PubMed] [Google Scholar]
- Williams M. Purine receptors in mammalian tissues: pharmacology and functional significance. Annu Rev Pharmacol Toxicol. 1987;27:315–345. doi: 10.1146/annurev.pa.27.040187.001531. [DOI] [PubMed] [Google Scholar]


