Abstract
1. Acetylcholine (ACh)-induced relaxation of aortic strips with endothelium and production of cyclic GMP between streptozotocin-induced diabetic and age-matched control rats were compared. 2. The concentration-response curve for ACh-induced relaxation was shifted to the right in diabetic rats. IC50 values for ACh were 4.57 +/- 0.67 x 10(-8) M and 1.00 +/- 0.87 x 10(-7) M in aortic strips from age-matched control and diabetic rats, respectively (n = 6, P less than 0.05). 3. Relaxations produced by atrial natriuretic peptide (ANP) in diabetic aortae were similar to those in age-matched vessels. 4. Relaxations produced by sodium nitroprusside (SNP) in diabetic aortae were similar to those in age-matched vessels. 5. Basal levels of cyclic GMP and ACh-induced production of cyclic GMP were significantly decreased in diabetic rats. 6. These results suggest that functional changes in endothelium but not in guanylate cyclase activity in the aorta may occur in diabetes, and thus, spontaneous and ACh-induced formation of cyclic GMP may be decreased. This decrease in production of cyclic GMP may be responsible for the decreased response of the aorta to the relaxant effect of ACh.
Full text
PDF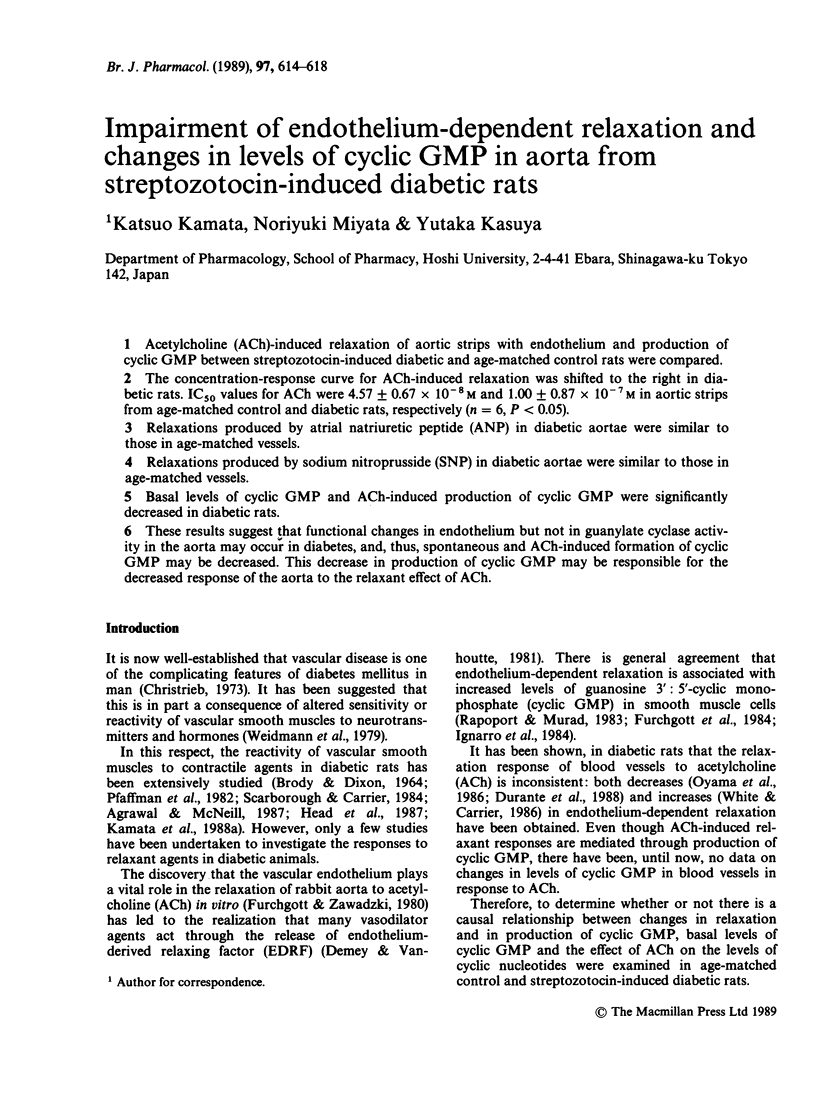



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal D. K., McNeill J. H. Vascular responses to agonists in rat mesenteric artery from diabetic rats. Can J Physiol Pharmacol. 1987 Jul;65(7):1484–1490. doi: 10.1139/y87-232. [DOI] [PubMed] [Google Scholar]
- BRODY M. J., DIXON R. L. VASCULAR REACTIVITY IN EXPERIMENTAL DIABETES MELLITUS. Circ Res. 1964 Jun;14:494–501. doi: 10.1161/01.res.14.6.494. [DOI] [PubMed] [Google Scholar]
- Cheng J. B., Shibata S. Vascular relaxation in the spontaneously hypertensive rat. J Cardiovasc Pharmacol. 1981 Sep-Oct;3(5):1126–1140. doi: 10.1097/00005344-198109000-00022. [DOI] [PubMed] [Google Scholar]
- Christlieb A. R. Diabetes and hypertensive vascular disease. Mechanisms and treatment. Am J Cardiol. 1973 Sep 20;32(4):592–606. doi: 10.1016/s0002-9149(73)80051-7. [DOI] [PubMed] [Google Scholar]
- DUBOWSKI K. M. An o-toluidine method for body-fluid glucose determination. Clin Chem. 1962 May-Jun;8:215–235. [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Role of the intima in cholinergic and purinergic relaxation of isolated canine femoral arteries. J Physiol. 1981 Jul;316:347–355. doi: 10.1113/jphysiol.1981.sp013792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante W., Sen A. K., Sunahara F. A. Impairment of endothelium-dependent relaxation in aortae from spontaneously diabetic rats. Br J Pharmacol. 1988 Jun;94(2):463–468. doi: 10.1111/j.1476-5381.1988.tb11548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Cherry P. D., Zawadzki J. V., Jothianandan D. Endothelial cells as mediators of vasodilation of arteries. J Cardiovasc Pharmacol. 1984;6 (Suppl 2):S336–S343. doi: 10.1097/00005344-198406002-00008. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Head R. J., Longhurst P. A., Panek R. L., Stitzel R. E. A contrasting effect of the diabetic state upon the contractile responses of aortic preparations from the rat and rabbit. Br J Pharmacol. 1987 Jun;91(2):275–286. doi: 10.1111/j.1476-5381.1987.tb10282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Burke T. M., Wood K. S., Wolin M. S., Kadowitz P. J. Association between cyclic GMP accumulation and acetylcholine-elicited relaxation of bovine intrapulmonary artery. J Pharmacol Exp Ther. 1984 Mar;228(3):682–690. [PubMed] [Google Scholar]
- Jayakody R. L., Senaratne M. P., Thomson A. B., Kappagoda C. T. Cholesterol feeding impairs endothelium-dependent relaxation of rabbit aorta. Can J Physiol Pharmacol. 1985 Sep;63(9):1206–1209. doi: 10.1139/y85-199. [DOI] [PubMed] [Google Scholar]
- Kamata K., Miyata N., Kasuya Y. Mechanisms of increased responses of the aorta to alpha-adrenoceptor agonists in streptozotocin-induced diabetic rats. J Pharmacobiodyn. 1988 Oct;11(10):707–713. doi: 10.1248/bpb1978.11.707. [DOI] [PubMed] [Google Scholar]
- Kamata K., Sakamoto A., Kasuya Y. Similarities between the relaxations induced by vasoactive intestinal peptide and by stimulation of the non-adrenergic non-cholinergic neurons in the rat stomach. Naunyn Schmiedebergs Arch Pharmacol. 1988 Oct;338(4):401–406. doi: 10.1007/BF00172117. [DOI] [PubMed] [Google Scholar]
- Konishi M., Su C. Role of endothelium in dilator responses of spontaneously hypertensive rat arteries. Hypertension. 1983 Nov-Dec;5(6):881–886. doi: 10.1161/01.hyp.5.6.881. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oyama Y., Kawasaki H., Hattori Y., Kanno M. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur J Pharmacol. 1986 Dec 2;132(1):75–78. doi: 10.1016/0014-2999(86)90013-0. [DOI] [PubMed] [Google Scholar]
- Pfaffman M. A., Ball C. R., Darby A., Hilman R. Insulin reversal of diabetes-induced inhibition of vascular contractility in the rat. Am J Physiol. 1982 Apr;242(4):H490–H495. doi: 10.1152/ajpheart.1982.242.4.H490. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Scarborough N. L., Carrier G. O. Nifedipine and alpha adrenoceptors in rat aorta. II. Role of extracellular calcium in enhanced alpha-2 adrenoceptor-mediated contraction in diabetes. J Pharmacol Exp Ther. 1984 Dec;231(3):603–609. [PubMed] [Google Scholar]
- Weidmann P., Beretta-Piccoli C., Keusch G., Glück Z., Mujagic M., Grimm M., Meier A., Ziegler W. H. Sodium-volume factor, cardiovascular reactivity and hypotensive mechanism of diuretic therapy in mild hypertension associated with diabetes mellitus. Am J Med. 1979 Nov;67(5):779–784. doi: 10.1016/0002-9343(79)90734-4. [DOI] [PubMed] [Google Scholar]
- White R. E., Carrier G. O. Supersensitivity and endothelium dependency of histamine-induced relaxation in mesenteric arteries isolated from diabetic rats. Pharmacology. 1986;33(1):34–38. doi: 10.1159/000138197. [DOI] [PubMed] [Google Scholar]
- Winquist R. J., Bunting P. B., Baskin E. P., Wallace A. A. Decreased endothelium-dependent relaxation in New Zealand genetic hypertensive rats. J Hypertens. 1984 Oct;2(5):541–545. doi: 10.1097/00004872-198410000-00015. [DOI] [PubMed] [Google Scholar]
- Winquist R. J., Faison E. P., Waldman S. A., Schwartz K., Murad F., Rapoport R. M. Atrial natriuretic factor elicits an endothelium-independent relaxation and activates particulate guanylate cyclase in vascular smooth muscle. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7661–7664. doi: 10.1073/pnas.81.23.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]


