Abstract
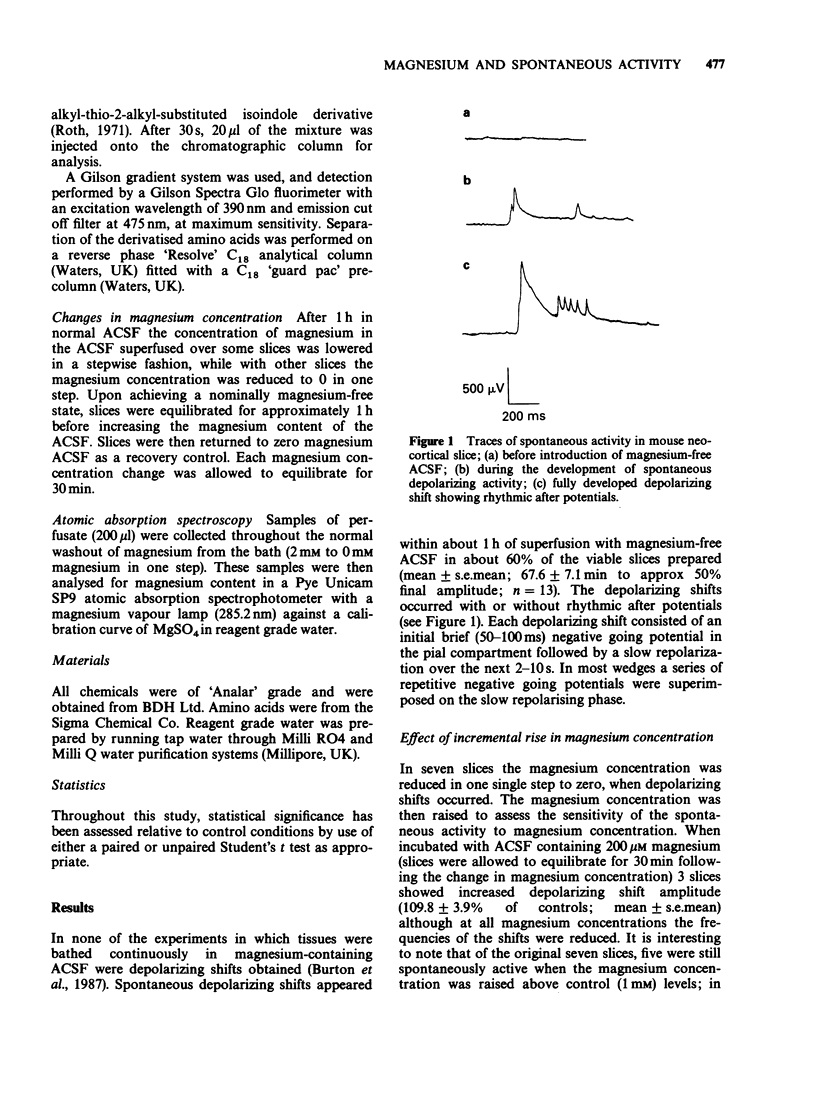
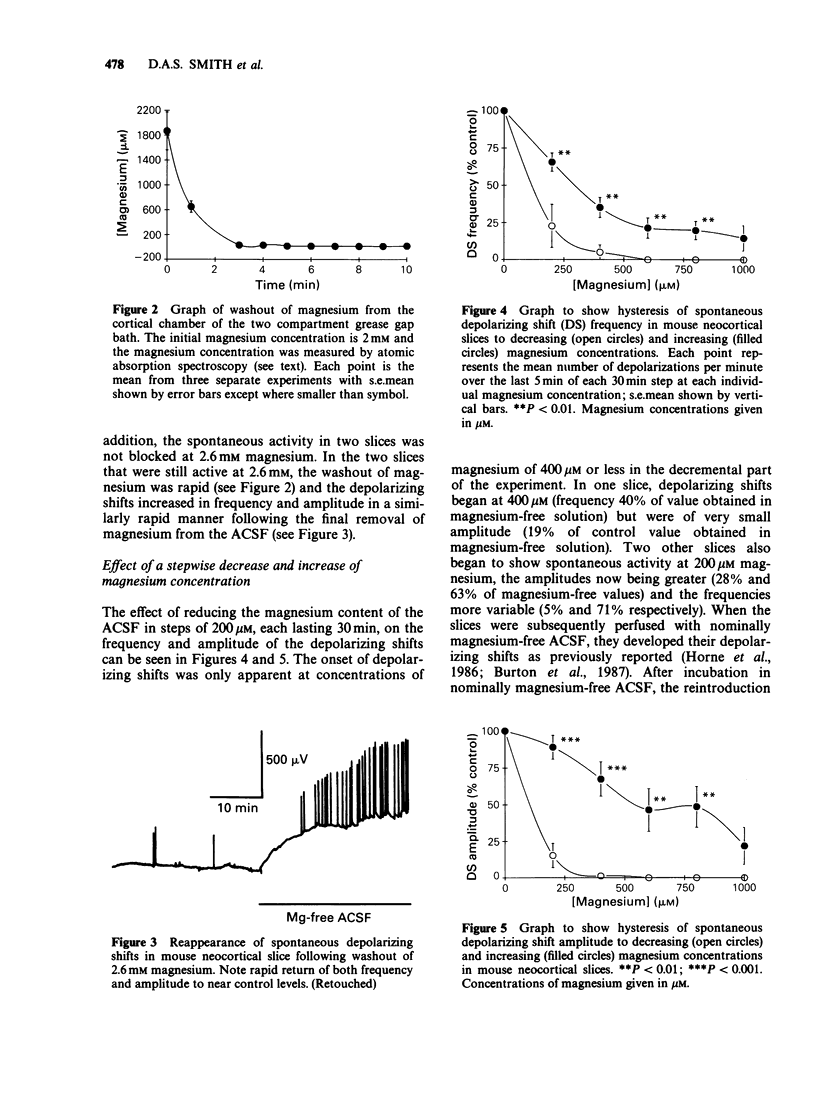
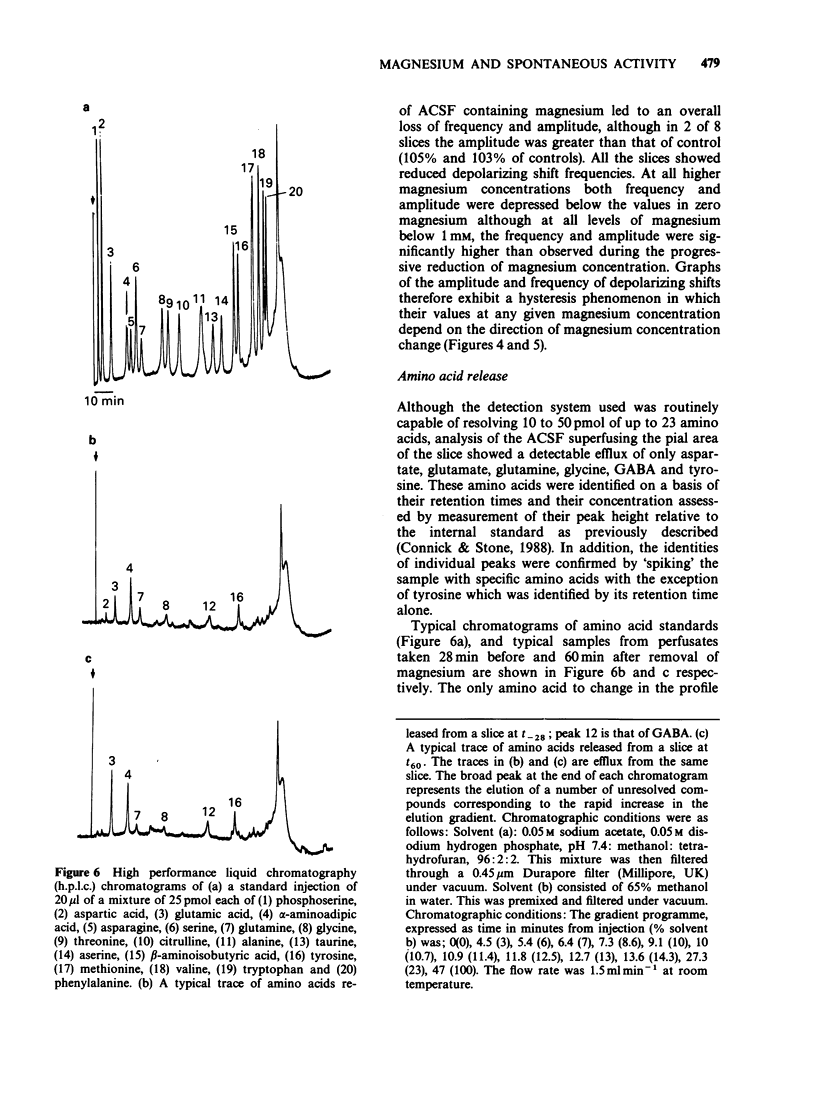
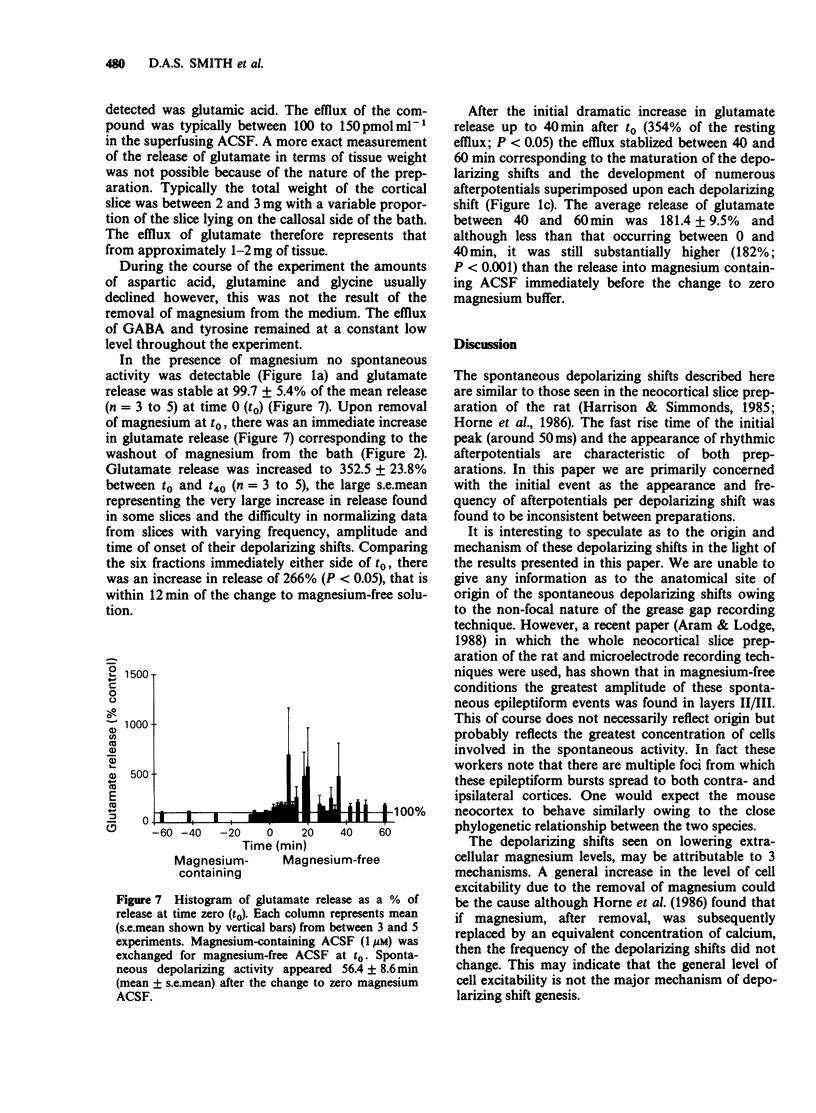
1. The mouse neocortical slice preparation, maintained in a two compartment, grease gap bath, exhibits spontaneous depolarizing activity (with or without rhythmic after potentials) after perfusion with magnesium-free artificial cerebrospinal fluid. 2. If the magnesium concentration is decrementally lowered over an extended time period, then incrementally raised following a similar time course, the spontaneous depolarizing shift activity shows a hysteresis (with regard to both frequency and amplitude), the depolarizing shifts being more resistant to magnesium during the incremental period. 3. The amino acid content of the perfusing fluid was analysed by high performance liquid chromatography (h.p.l.c.). Although a basal efflux of 6 amino acids was quantifiable, only glutamate levels increased following superfusion of the preparation with magnesium-free, artificial cerebrospinal fluid. 4. Glutamate release increased to 266% of the resting release in the presence of magnesium within the first 12 min of the change into magnesium-free artificial cerebrospinal fluid. This increase in release preceded the onset of spontaneous depolarising activity. The release of glutamate remained elevated at 182% of control up to 60 min after perfusion with magnesium-free buffer, when depolarizing activity was well established. 5. A model is presented and discussed for the genesis and maintenance of the spontaneous depolarizing shifts. It is suggested that the maintenance of this spontaneous activity reflects a long term enhancement of neocortical neurone excitability which may be related to long term potentiation in the hippocampus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. W., Lewis D. V., Swartzwelder H. S., Wilson W. A. Magnesium-free medium activates seizure-like events in the rat hippocampal slice. Brain Res. 1986 Nov 19;398(1):215–219. doi: 10.1016/0006-8993(86)91274-6. [DOI] [PubMed] [Google Scholar]
- Aram J. A., Lodge D. Validation of a neocortical slice preparation for the study of epileptiform activity. J Neurosci Methods. 1988 Apr;23(3):211–224. doi: 10.1016/0165-0270(88)90005-2. [DOI] [PubMed] [Google Scholar]
- Artola A., Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987 Dec 17;330(6149):649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Avoli M., Louvel J., Pumain R., Olivier A. Seizure-like discharges induced by lowering [Mg2+]o in the human epileptogenic neocortex maintained in vitro. Brain Res. 1987 Aug 4;417(1):199–203. doi: 10.1016/0006-8993(87)90201-0. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton N. R., Smith D. A., Stone T. W. The mouse neocortical slice: preparation and responses to excitatory amino acids. Comp Biochem Physiol C. 1987;88(1):47–55. doi: 10.1016/0742-8413(87)90045-4. [DOI] [PubMed] [Google Scholar]
- Coan E. J., Collingridge G. L. Characterization of an N-methyl-D-aspartate receptor component of synaptic transmission in rat hippocampal slices. Neuroscience. 1987 Jul;22(1):1–8. doi: 10.1016/0306-4522(87)90192-8. [DOI] [PubMed] [Google Scholar]
- Connick J. H., Stone T. W. Quinolinic acid effects on amino acid release from the rat cerebral cortex in vitro and in vivo. Br J Pharmacol. 1988 Apr;93(4):868–876. doi: 10.1111/j.1476-5381.1988.tb11474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drejer J., Honoré T., Schousboe A. Excitatory amino acid-induced release of 3H-GABA from cultured mouse cerebral cortex interneurons. J Neurosci. 1987 Sep;7(9):2910–2916. doi: 10.1523/JNEUROSCI.07-09-02910.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Watkins J. C. Selective antagonism by Mg2+ of amino acid-induced depolarization of spinal neurones. Experientia. 1977 Apr 15;33(4):489–491. doi: 10.1007/BF01922227. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Simmonds M. A. Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. Br J Pharmacol. 1985 Feb;84(2):381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer E. J., Nowak L. M., Macdonald R. L. Membrane depolarization and prolongation of calcium-dependent action potentials of mouse neurons in cell culture by two convulsants: bicuculline and penicillin. Brain Res. 1982 Jan 28;232(1):41–56. doi: 10.1016/0006-8993(82)90609-6. [DOI] [PubMed] [Google Scholar]
- Horne A. L., Harrison N. L., Turner J. P., Simmonds M. A. Spontaneous paroxysmal activity induced by zero magnesium and bicuculline: suppression by NMDA antagonists and GABA mimetics. Eur J Pharmacol. 1986 Mar 18;122(2):231–238. doi: 10.1016/0014-2999(86)90107-x. [DOI] [PubMed] [Google Scholar]
- Huang Y. Y., Wigström H., Gustafsson B. Facilitated induction of hippocampal long-term potentiation in slices perfused with low concentrations of magnesium. Neuroscience. 1987 Jul;22(1):9–16. doi: 10.1016/0306-4522(87)90193-x. [DOI] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- McLennan H. Receptors for the excitatory amino acids in the mammalian central nervous system. Prog Neurobiol. 1983;20(3-4):251–271. doi: 10.1016/0301-0082(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Richards C. D., Sercombe R. Calcium, magnesium and the electrical activity of guinea-pig olfactory coex in vitro. J Physiol. 1970 Dec;211(3):571–584. doi: 10.1113/jphysiol.1970.sp009294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. Fluorescence reaction for amino acids. Anal Chem. 1971 Jun;43(7):880–882. doi: 10.1021/ac60302a020. [DOI] [PubMed] [Google Scholar]
- Rothman S. M., Olney J. W. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986 Feb;19(2):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- Skrede K. K., Malthe-Sørenssen D. Increased resting and evoked release of transmitter following repetitive electrical tetanization in hippocampus: a biochemical correlate to long-lasting synaptic potentiation. Brain Res. 1981 Mar 16;208(2):436–441. doi: 10.1016/0006-8993(81)90573-4. [DOI] [PubMed] [Google Scholar]
- Stone T. W., Burton N. R. NMDA receptors and ligands in the vertebrate CNS. Prog Neurobiol. 1988;30(4):333–368. doi: 10.1016/0301-0082(88)90027-5. [DOI] [PubMed] [Google Scholar]
- Stone T. W., Connick J. H. Quinolinic acid and other kynurenines in the central nervous system. Neuroscience. 1985 Jul;15(3):597–617. doi: 10.1016/0306-4522(85)90063-6. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C. N-methylaspartate receptors mediate epileptiform activity evoked in some, but not all, conditions in rat neocortical slices. Neuroscience. 1986 Dec;19(4):1161–1177. doi: 10.1016/0306-4522(86)90130-2. [DOI] [PubMed] [Google Scholar]
- Turnell D. C., Cooper J. D. Rapid assay for amino acids in serum or urine by pre-column derivatization and reversed-phase liquid chromatography. Clin Chem. 1982 Mar;28(3):527–531. [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]



