Abstract
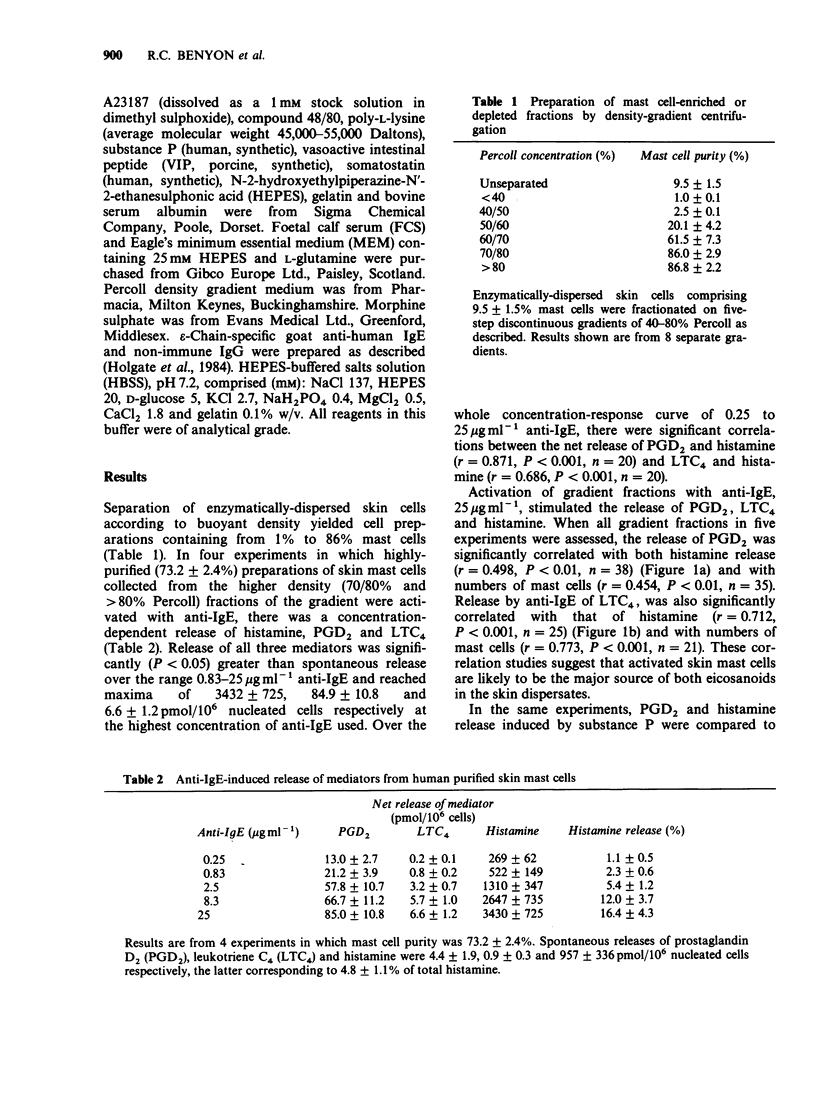
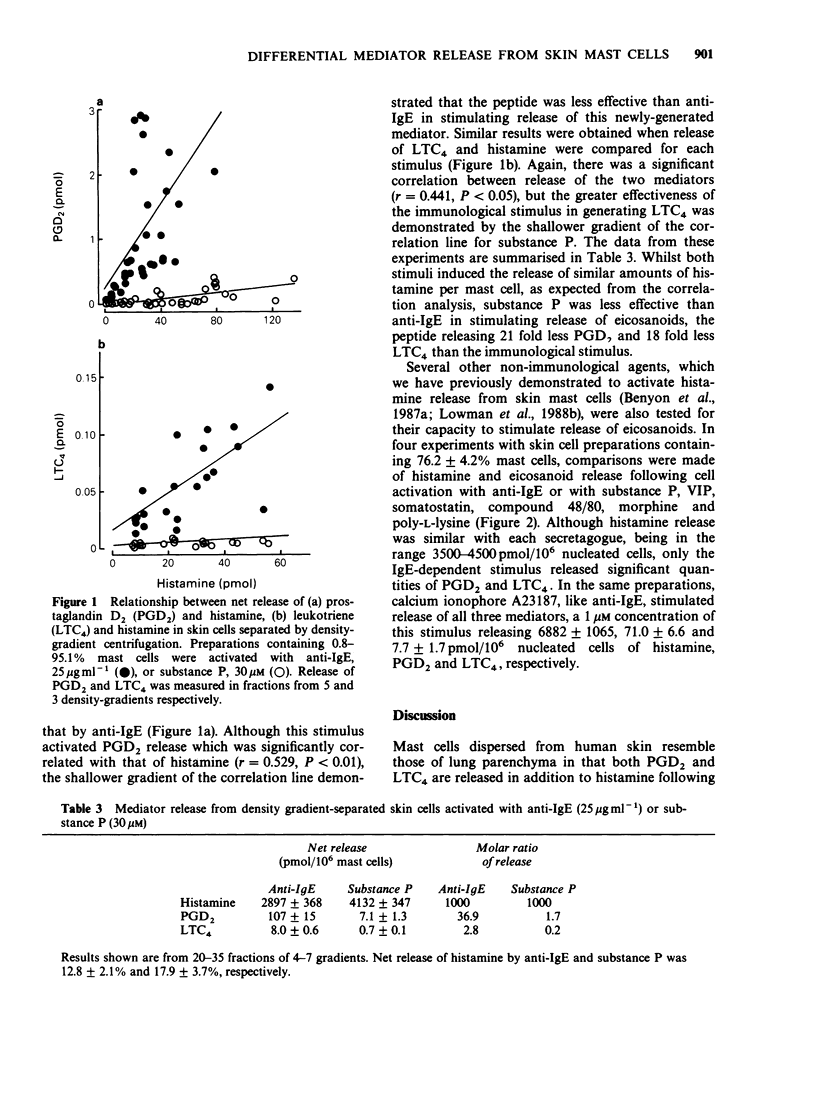
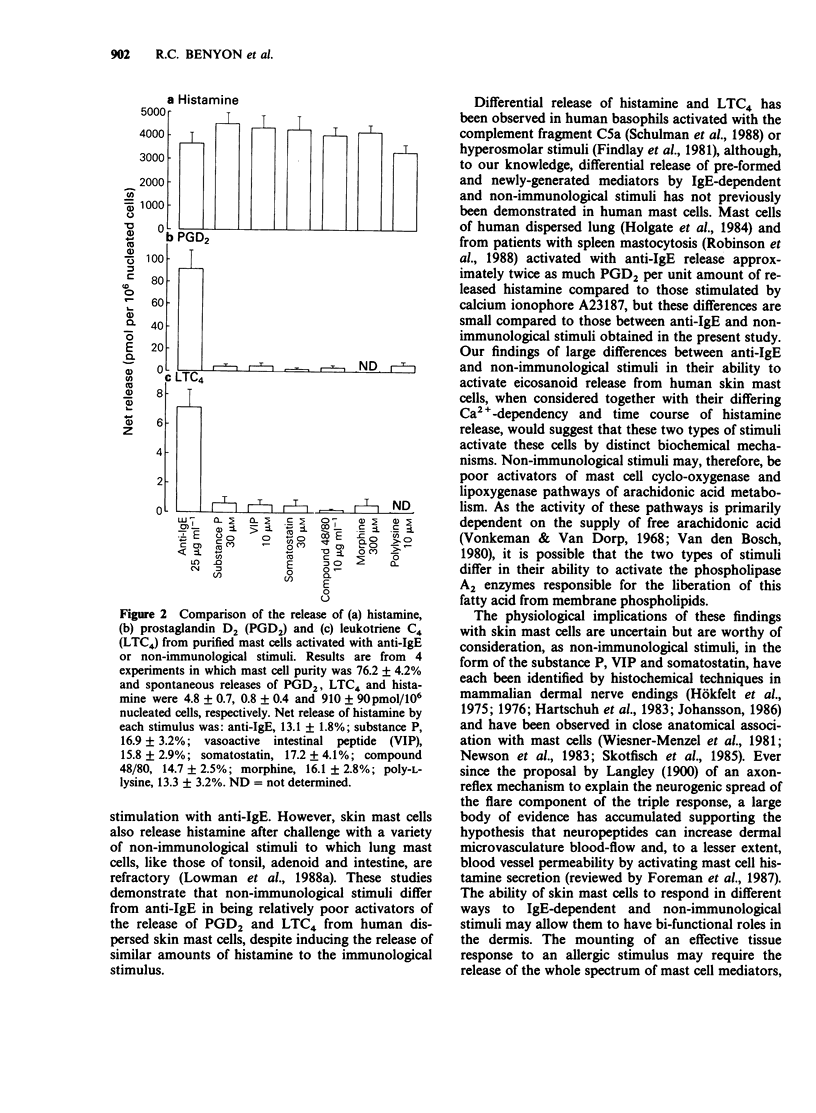
1. Cells were dispersed from human foreskin using a mixture of collagenase and hyaluronidase and separated into mast cell-depleted (less than 1%) or enriched (greater than 75%) preparations by density-gradient centrifugation. 2. Challenge of gradient fractions with epsilon-chain-specific anti-human IgE stimulated the release of histamine, prostaglandin D2 (PGD2) and leukotriene C4 (LTC4). The release of eicosanoids was significantly correlated with that of histamine, suggesting that they are derived from the mast cell population of the dispersate. In highly purified (76.2 +/- 4.2%) mast cell preparations, maximum net release of histamine, PGD2 and LTC4 was 3432 +/- 725, 84.9 +/- 10.8 and 6.6 +/- 1.2 pmol/10(6) nucleated cells. 3. The non-immunological stimuli substance P, vasoactive intestinal peptide (VIP), somatostatin, compound 48/80, morphine and poly-L-lysine released similar amounts of histamine to anti-IgE, but 12 to 21 fold less PGD2 and LTC4. 4. These studies suggest that IgE-dependent and non-immunological stimuli activate human skin mast cells by different secretory mechanisms, a hypothesis supported by our previous findings of differences in Ca2+ requirements and time-course of histamine release. Activation by the non-immunological mechanism may be of importance in vivo due to the close anatomical association between skin mast cells and dermal nerve-terminals containing neuropeptides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benyon R. C., Church M. K., Clegg L. S., Holgate S. T. Dispersion and characterisation of mast cells from human skin. Int Arch Allergy Appl Immunol. 1986;79(3):332–334. doi: 10.1159/000233996. [DOI] [PubMed] [Google Scholar]
- Benyon R. C., Lowman M. A., Church M. K. Human skin mast cells: their dispersion, purification, and secretory characterization. J Immunol. 1987 Feb 1;138(3):861–867. [PubMed] [Google Scholar]
- Benyon R. C., Robinson C., Holgate S. T., Church M. K. Prostaglandin D2 release from human skin mast cells in response to ionophore A23187. Br J Pharmacol. 1987 Nov;92(3):635–638. doi: 10.1111/j.1476-5381.1987.tb11366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard H., Kristensen J., Søndergaard J. The effect of leukotriene C4 and D4 on cutaneous blood flow in humans. Prostaglandins. 1982 Jun;23(6):797–801. doi: 10.1016/0090-6980(82)90124-1. [DOI] [PubMed] [Google Scholar]
- Camp R. D., Coutts A. A., Greaves M. W., Kay A. B., Walport M. J. Responses of human skin to intradermal injection of leukotrienes C4, D4 and B4. Br J Pharmacol. 1983 Nov;80(3):497–502. doi: 10.1111/j.1476-5381.1983.tb10721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church M. K., Pao G. J., Holgate S. T. Characterization of histamine secretion from mechanically dispersed human lung mast cells: effects of anti-IgE, calcium ionophore A23187, compound 48/80, and basic polypeptides. J Immunol. 1982 Nov;129(5):2116–2121. [PubMed] [Google Scholar]
- Findlay S. R., Dvorak A. M., Kagey-Sobotka A., Lichtenstein L. M. Hyperosmolar triggering of histamine release from human basophils. J Clin Invest. 1981 Jun;67(6):1604–1613. doi: 10.1172/JCI110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C. Neuropeptides and the pathogenesis of allergy. Allergy. 1987 Jan;42(1):1–11. doi: 10.1111/j.1398-9995.1987.tb02180.x. [DOI] [PubMed] [Google Scholar]
- Hartschuh W., Weihe E., Reinecke M. Peptidergic (neurotensin, VIP, substance P) nerve fibres in the skin. Immunohistochemical evidence of an involvement of neuropeptides in nociception, pruritus and inflammation. Br J Dermatol. 1983 Jul;109 (Suppl 25):14–17. doi: 10.1111/j.1365-2133.1983.tb06811.x. [DOI] [PubMed] [Google Scholar]
- Holgate S. T., Burns G. B., Robinson C., Church M. K. Anaphylactic- and calcium-dependent generation of prostaglandin D2 (PGD2), thromboxane B2, and other cyclooxygenase products of arachidonic acid by dispersed human lung cells and relationship to histamine release. J Immunol. 1984 Oct;133(4):2138–2144. [PubMed] [Google Scholar]
- Hökfelt T., Elde R., Johansson O., Luft R., Nilsson G., Arimura A. Immunohistochemical evidence for separate populations of somatostatin-containing and substance P-containing primary afferent neurons in the rat. Neuroscience. 1976;1(2):131–136. doi: 10.1016/0306-4522(76)90008-7. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res. 1975 Dec 19;100(2):235–252. doi: 10.1016/0006-8993(75)90481-3. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Induction of erythema-wheal reactions by soluble antigen-gammaE antibody complexes in humans. J Immunol. 1968 Jul;101(1):68–78. [PubMed] [Google Scholar]
- Johansson O. A detailed account of NPY-immunoreactive nerves and cells of the human skin. Comparison with VIP-, substance P- and PHI-containing structures. Acta Physiol Scand. 1986 Oct;128(2):147–153. doi: 10.1111/j.1748-1716.1986.tb07961.x. [DOI] [PubMed] [Google Scholar]
- Kimura I., Moritani Y., Tanizaki Y. Basophils in bronchial asthma with reference to reagin-type allergy. Clin Allergy. 1973 Jun;3(2):195–202. doi: 10.1111/j.1365-2222.1973.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Langley J. N. On axon-reflexes in the pre-ganglionic fibres of the sympathetic system. J Physiol. 1900 Aug 29;25(5):364–398. doi: 10.1113/jphysiol.1900.sp000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman M. A., Benyon R. C., Church M. K. Characterization of neuropeptide-induced histamine release from human dispersed skin mast cells. Br J Pharmacol. 1988 Sep;95(1):121–130. doi: 10.1111/j.1476-5381.1988.tb16555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman M. A., Rees P. H., Benyon R. C., Church M. K. Human mast cell heterogeneity: histamine release from mast cells dispersed from skin, lung, adenoids, tonsils, and colon in response to IgE-dependent and nonimmunologic stimuli. J Allergy Clin Immunol. 1988 Mar;81(3):590–597. [PubMed] [Google Scholar]
- Marks R. M., Roche W. R., Czerniecki M., Penny R., Nelson D. S. Mast cell granules cause proliferation of human microvascular endothelial cells. Lab Invest. 1986 Sep;55(3):289–294. [PubMed] [Google Scholar]
- Newson B., Dahlström A., Enerbäck L., Ahlman H. Suggestive evidence for a direct innervation of mucosal mast cells. Neuroscience. 1983 Oct;10(2):565–570. doi: 10.1016/0306-4522(83)90153-7. [DOI] [PubMed] [Google Scholar]
- Peters S. P., Kagey-Sobotka A., MacGlashan D. W., Jr, Lichtenstein L. M. Effect of prostaglandin D2 in modulating histamine release from human basophils. J Pharmacol Exp Ther. 1984 Feb;228(2):400–406. [PubMed] [Google Scholar]
- Peters S. P., MacGlashan D. W., Jr, Schulman E. S., Schleimer R. P., Hayes E. C., Rokach J., Adkinson N. F., Jr, Lichtenstein L. M. Arachidonic acid metabolism in purified human lung mast cells. J Immunol. 1984 Apr;132(4):1972–1979. [PubMed] [Google Scholar]
- Rees P. H., Hillier K., Church M. K. The secretory characteristics of mast cells isolated from the human large intestinal mucosa and muscle. Immunology. 1988 Nov;65(3):437–442. [PMC free article] [PubMed] [Google Scholar]
- Robinson C., Benyon R. C., Agius R. M., Jones D. B., Wright D. H., Holgate S. T. The immunoglobulin E- and calcium-dependent release of histamine and eicosanoids from human dispersed mastocytosis spleen cells. J Invest Dermatol. 1988 Mar;90(3):359–365. doi: 10.1111/1523-1747.ep12456379. [DOI] [PubMed] [Google Scholar]
- SCHAYER R. W. Evidence that induced histamine is an intrinsic regulator of the microcirculatory system. Am J Physiol. 1962 Jan;202:66–72. doi: 10.1152/ajplegacy.1962.202.1.66. [DOI] [PubMed] [Google Scholar]
- Schulman E. S., Post T. J., Henson P. M., Giclas P. C. Differential effects of the complement peptides, C5a and C5a des Arg on human basophil and lung mast cell histamine release. J Clin Invest. 1988 Mar;81(3):918–923. doi: 10.1172/JCI113403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skofitsch G., Savitt J. M., Jacobowitz D. M. Suggestive evidence for a functional unit between mast cells and substance P fibers in the rat diaphragm and mesentery. Histochemistry. 1985;82(1):5–8. doi: 10.1007/BF00502084. [DOI] [PubMed] [Google Scholar]
- Soter N. A., Lewis R. A., Corey E. J., Austen K. F. Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. J Invest Dermatol. 1983 Feb;80(2):115–119. doi: 10.1111/1523-1747.ep12531738. [DOI] [PubMed] [Google Scholar]
- Subba Rao P. V., Friedman M. M., Atkins F. M., Metcalfe D. D. Phagocytosis of mast cell granules by cultured fibroblasts. J Immunol. 1983 Jan;130(1):341–349. [PubMed] [Google Scholar]
- Vonkeman H., van Dorp D. A. The action of prostaglandin synthetase on 2-arachidonyl-lecithin. Biochim Biophys Acta. 1968 Oct 22;164(2):430–432. doi: 10.1016/0005-2760(68)90169-0. [DOI] [PubMed] [Google Scholar]
- Wiesner-Menzel L., Schulz B., Vakilzadeh F., Czarnetzki B. M. Electron microscopical evidence for a direct contact between nerve fibres and mast cells. Acta Derm Venereol. 1981;61(6):465–469. doi: 10.2340/0001555561465469. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980 Sep 30;604(2):191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]



