Abstract
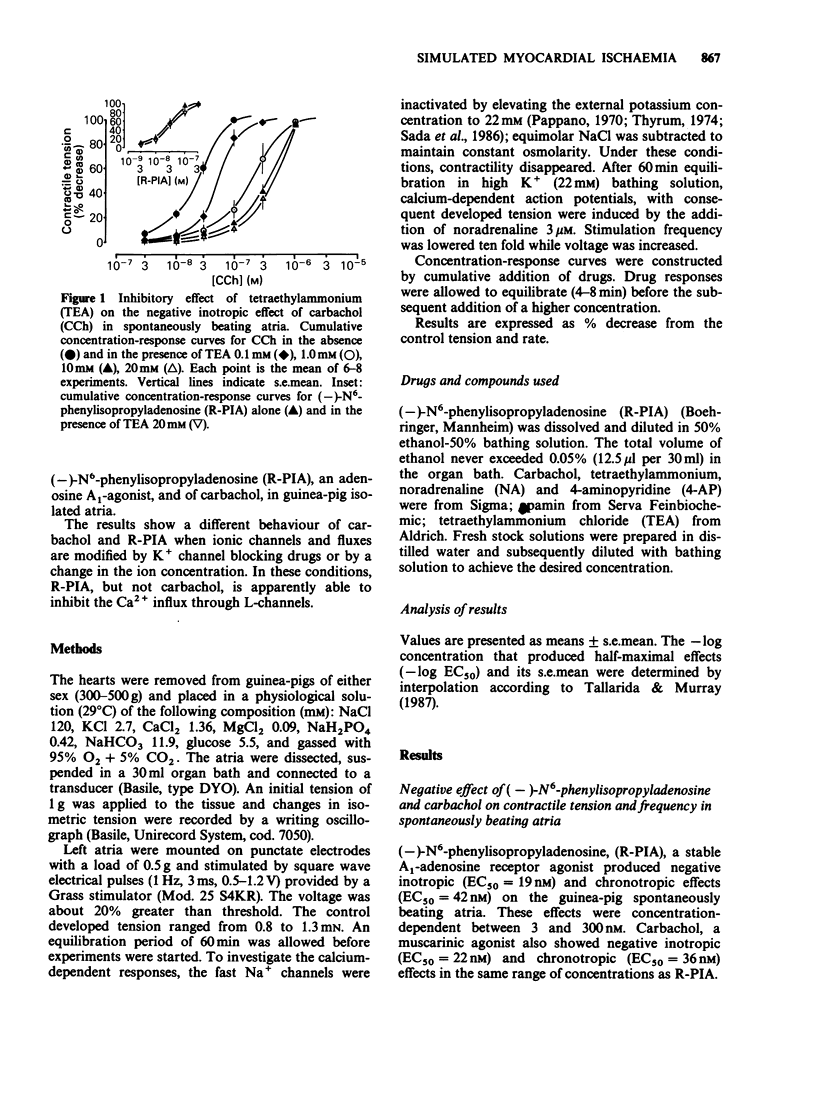
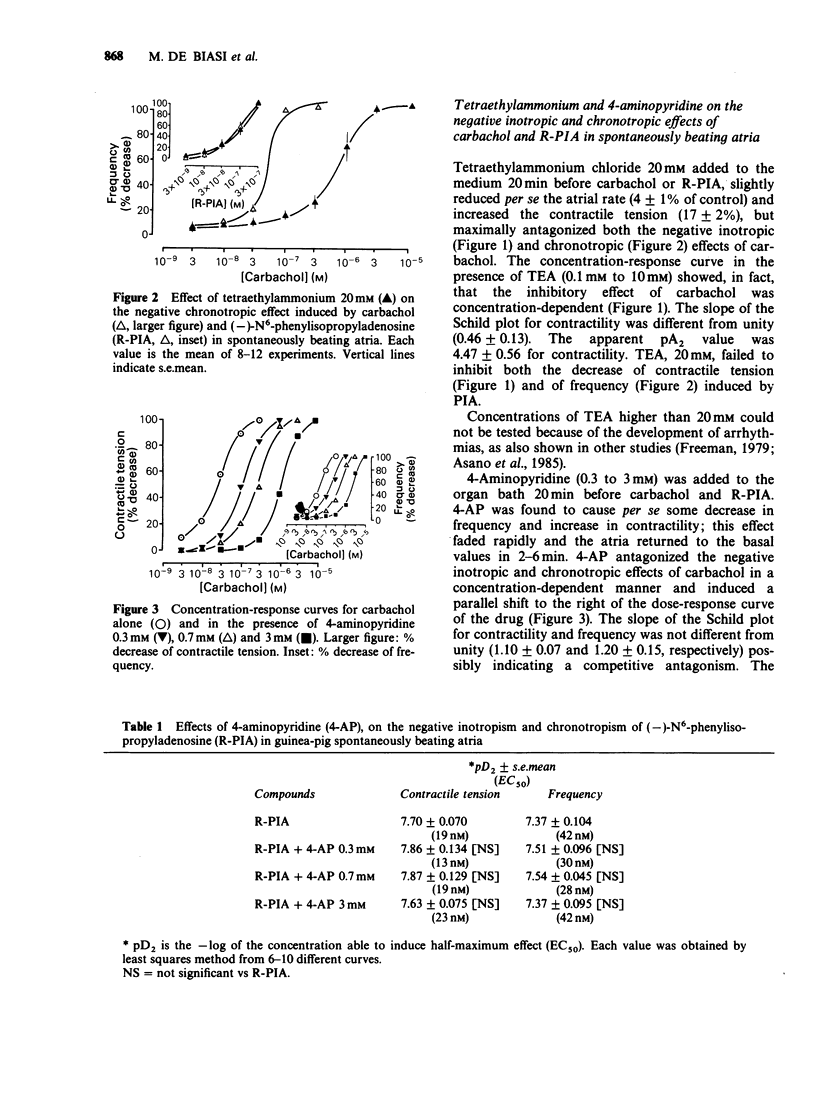
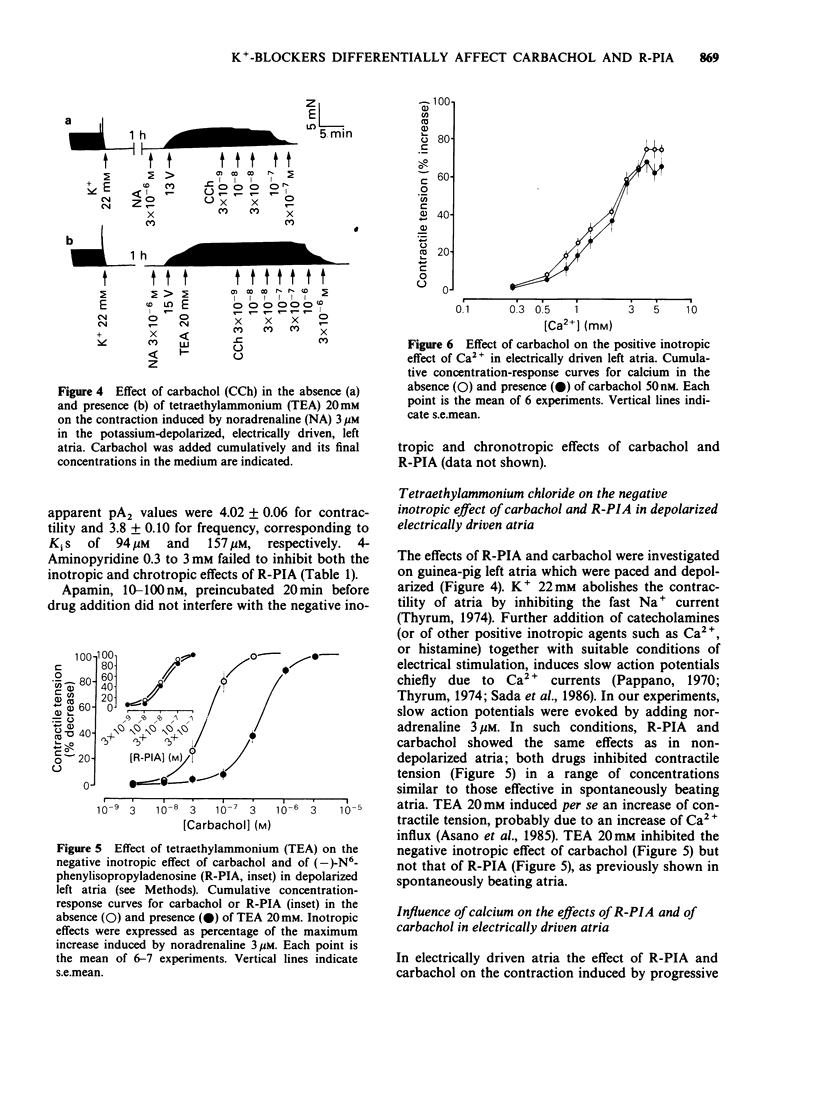
1. The effect of three different potassium channel blockers (tetraethylammonium, TEA; 4-aminopyridine, 4-AP; and apamin) and of variations in the concentration of K+ and Ca2+ in the medium, have been studied on the responses of guinea-pig isolated atria to (-)-N6-phenylisopropyladenosine (R-PIA), a stable adenosine A1-receptor agonist, and to carbachol, a muscarinic agonist. R-PIA and carbachol showed the same negative inotropic effects over a similar range of concentrations (3-300 microM), both in spontaneously beating and in electrically driven atria. 2. TEA (0.1 to 20 mM) and 4-AP (0.3 to 3 mM), both antagonized the negative inotropic and chronotropic effects of carbachol in a concentration-dependent manner. In contrast, these compounds failed to inhibit the effects induced by R-PIA. Apamin, a specific blocker of a low conductance Ca2+-activated K+ channel, was ineffective in accordance with the absence of these channels in atrial tissue. 3. TEA (0.1 to 20mM) inhibited the negative inotropic effect of carbachol, but not that of R-PIA, in atria paced and depolarized by a high K+ medium (22 mM). In this preparation Na+ current is abolished and the contraction induced by noradrenaline and electrical stimulation is solely dependent on Ca2+ influx currents. 4. Stepwise addition of Ca2+ to a calcium-depleted perfusing medium of electrically driven atria, induced a positive inotropic effect which was inhibited by R-PIA. In contrast, carbachol had no effect. 5. In agreement with our previous study, the data suggest that R-PIA acts on isolated atria by inhibiting Ca2+ influx through L-channels.
Full text
PDF
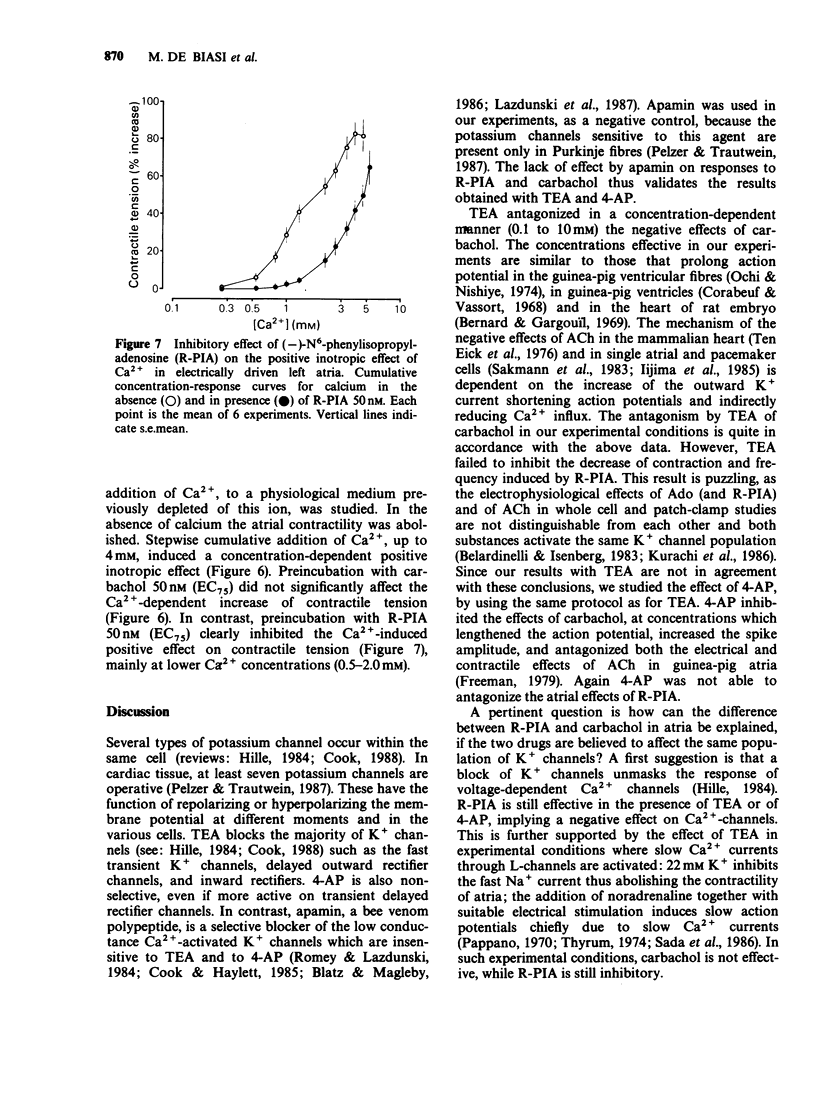


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Shigenobu K., Kasuya Y. TEA prevents the decline of the duration of the action potential in hypoxic cardiac muscle. Jpn J Pharmacol. 1985 May;38(1):65–72. doi: 10.1254/jjp.38.65. [DOI] [PubMed] [Google Scholar]
- Belardinelli L., Isenberg G. Isolated atrial myocytes: adenosine and acetylcholine increase potassium conductance. Am J Physiol. 1983 May;244(5):H734–H737. doi: 10.1152/ajpheart.1983.244.5.H734. [DOI] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Borea P. A., Caparrotta L., De Biasi M., Fassina G., Froldi G., Pandolfo L., Ragazzi E. Effect of selective agonists and antagonists on atrial adenosine receptors and their interaction with Bay K 8644 and [3H]-nitrendipine. Br J Pharmacol. 1989 Feb;96(2):372–378. doi: 10.1111/j.1476-5381.1989.tb11827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm M., Brückner R., Neumann J., Schmitz W., Scholz H., Starbatty J. Role of guanine nucleotide-binding protein in the regulation by adenosine of cardiac potassium conductance and force of contraction. Evaluation with pertussis toxin. Naunyn Schmiedebergs Arch Pharmacol. 1986 Apr;332(4):403–405. doi: 10.1007/BF00500095. [DOI] [PubMed] [Google Scholar]
- Caparrotta L., Fassina G., Froldi G., Poja R. Antagonism between (-)-N6-phenylisopropyladenosine and the calcium channel facilitator Bay K 8644, on guinea-pig isolated atria. Br J Pharmacol. 1987 Jan;90(1):23–30. doi: 10.1111/j.1476-5381.1987.tb16821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerbai E., Klöckner U., Isenberg G. The alpha subunit of the GTP binding protein activates muscarinic potassium channels of the atrium. Science. 1988 Jun 24;240(4860):1782–1783. doi: 10.1126/science.2454511. [DOI] [PubMed] [Google Scholar]
- Cook N. S., Haylett D. G. Effects of apamin, quinine and neuromuscular blockers on calcium-activated potassium channels in guinea-pig hepatocytes. J Physiol. 1985 Jan;358:373–394. doi: 10.1113/jphysiol.1985.sp015556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. S. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988 Jan;9(1):21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- Coraboeuf E., Vassort G. Effects of some inhibitors of ionic permeabilities on ventricular action potential and contraction of rat and guinea-pig hearts. J Electrocardiol. 1968;1(1):19–29. doi: 10.1016/s0022-0736(68)80005-6. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Forda S. R., Scott R. H. Calcium-dependent currents in cultured rat dorsal root ganglion neurones are inhibited by an adenosine analogue. J Physiol. 1986 Apr;373:47–61. doi: 10.1113/jphysiol.1986.sp016034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S. E. Cholinergic mechanisms in heart: interactions with 4-aminopyridine. J Pharmacol Exp Ther. 1979 Jul;210(1):7–14. [PubMed] [Google Scholar]
- Hartzell H. C. Adenosine receptors in frog sinus venosus: slow inhibitory potentials produced by adenine compounds and acetylcholine. J Physiol. 1979 Aug;293:23–49. doi: 10.1113/jphysiol.1979.sp012877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T., Irisawa H., Kameyama M. Membrane currents and their modification by acetylcholine in isolated single atrial cells of the guinea-pig. J Physiol. 1985 Feb;359:485–501. doi: 10.1113/jphysiol.1985.sp015598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON E. A., MCKINNON M. G. Effect of acetylcholine and adenosine on cardiac cellular potentials. Nature. 1956 Nov 24;178(4543):1174–1175. doi: 10.1038/1781174a0. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- Ochi R., Nishiye H. Effect of intracellular tetraethylammonium ion on action potential in the guinea-pig's myocardium. Pflugers Arch. 1974 May 6;348(4):305–316. doi: 10.1007/BF00589220. [DOI] [PubMed] [Google Scholar]
- Pappano A. J. Calcium-dependent action potentials produced by catecholamines in guinea pig atrial muscle fibers depolarized by potassium. Circ Res. 1970 Sep;27(3):379–390. doi: 10.1161/01.res.27.3.379. [DOI] [PubMed] [Google Scholar]
- Pelzer D., Trautwein W. Currents through ionic channels in multicellular cardiac tissue and single heart cells. Experientia. 1987 Dec 1;43(11-12):1153–1162. doi: 10.1007/BF01945515. [DOI] [PubMed] [Google Scholar]
- Romey G., Lazdunski M. The coexistence in rat muscle cells of two distinct classes of Ca2+-dependent K+ channels with different pharmacological properties and different physiological functions. Biochem Biophys Res Commun. 1984 Jan 30;118(2):669–674. doi: 10.1016/0006-291x(84)91355-x. [DOI] [PubMed] [Google Scholar]
- Sada H., Sada S., Sperelakis N. Recovery of the slow action potential is hastened by the calcium slow channel agonist, Bay-K-8644. Eur J Pharmacol. 1986 Jan 14;120(1):17–24. doi: 10.1016/0014-2999(86)90634-5. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Noma A., Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983 May 19;303(5914):250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Ten Eick R., Nawrath H., McDonald T. F., Trautwein W. On the mechanism of the negative inotropic effect of acetylcholine. Pflugers Arch. 1976 Feb 24;361(3):207–213. doi: 10.1007/BF00587284. [DOI] [PubMed] [Google Scholar]
- Thyrum P. T. Inotropic stimuli and systolic transmembrane calcium flow in depolarized guinea-pig atria. J Pharmacol Exp Ther. 1974 Jan;188(1):166–179. [PubMed] [Google Scholar]
- West G. A., Belardinelli L. Correlation of sinus slowing and hyperpolarization caused by adenosine in sinus node. Pflugers Arch. 1985 Jan;403(1):75–81. doi: 10.1007/BF00583285. [DOI] [PubMed] [Google Scholar]


