Abstract
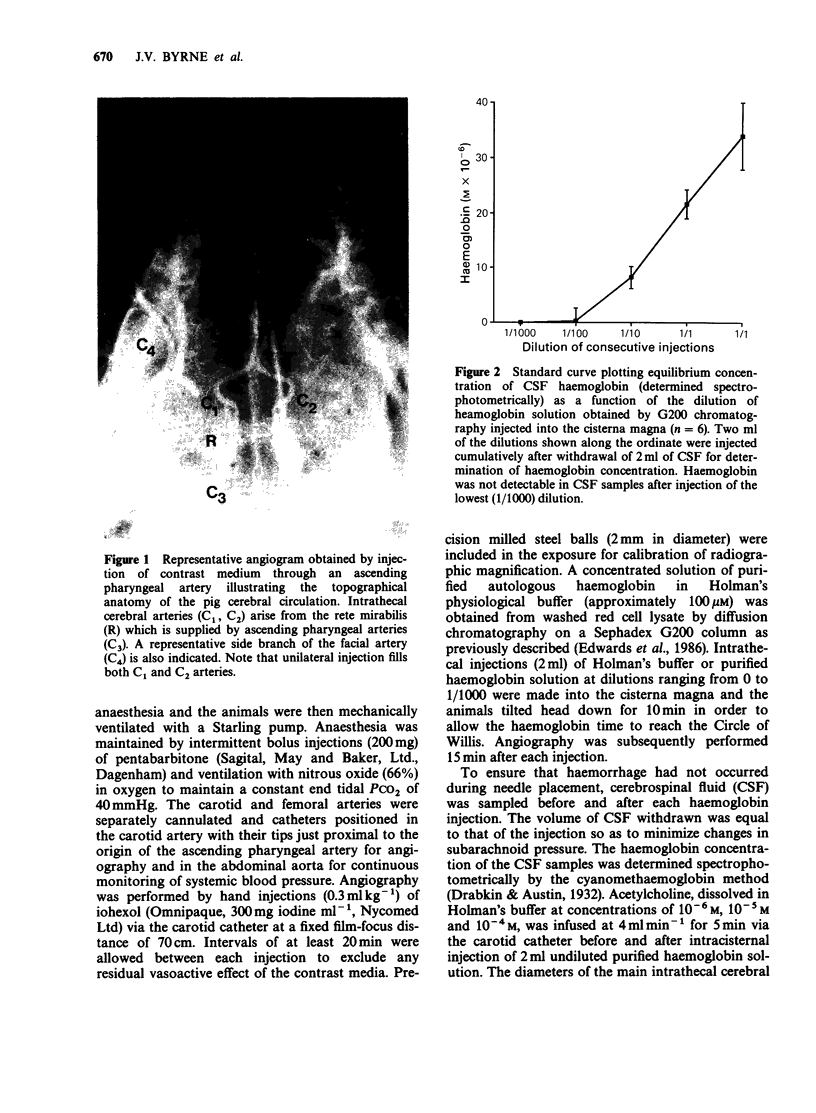
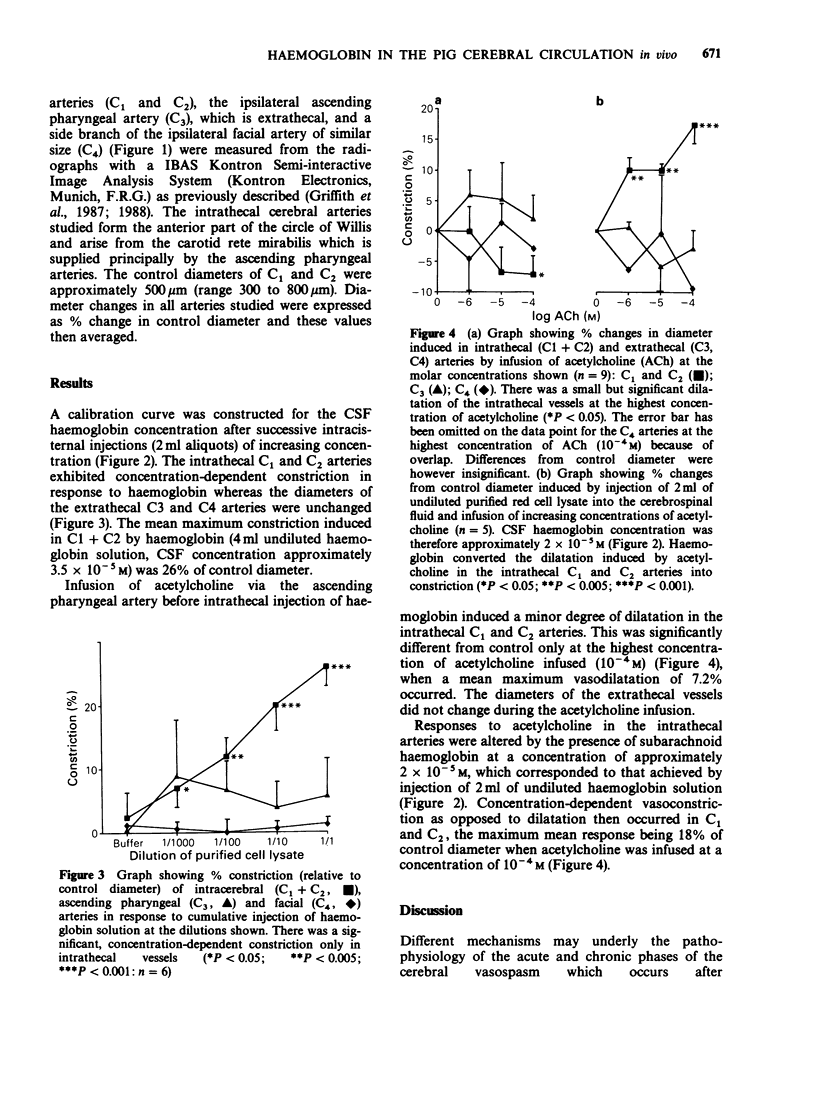
1. Angiographic techniques have been used to study the influence of intracisternally injected haemoglobin on the diameters of the main intrathecal and representative extrathecal (ascending pharyngeal and facial) cranial arteries of the anaesthetized pig. 2. Intracisternal injection of haemoglobin caused concentration-dependent decreases in the diameters of intra- but not extrathecal arteries suggesting that haemoglobin possesses local vasoconstrictor activity. 3. When infused into one ascending pharyngeal artery, acetylcholine (ACh) caused slight dilatation of the intrathecal arteries but no change in the diameters of the ascending pharyngeal and facial arteries. The dilator response induced by ACh in the intrathecal arteries was converted into frank constriction after intracisternal injection of haemoglobin (cerebrospinal fluid concentration approximately 2 x 10(-5) M). 4. These findings are consistent with the hypothesis that subarachnoid haemoglobin can induce cerebral artery constriction by acting as an extraluminal 'sink' for intimally released endothelium-derived relaxing factor (EDRF) and may be relevant to the pathogenesis of vasospasm after subarachnoid haemorrhage in man.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen R. A., Shepherd J. T., Vanhoutte P. M. Inhibitory role of the endothelium in the response of isolated coronary arteries to platelets. Science. 1983 Jul 15;221(4607):273–274. doi: 10.1126/science.6574604. [DOI] [PubMed] [Google Scholar]
- Collins P., Chappell S. P., Griffith T. M., Lewis M. J., Henderson A. H. Differences in basal endothelium-derived relaxing factor activity in different artery types. J Cardiovasc Pharmacol. 1986 Nov-Dec;8(6):1158–1162. doi: 10.1097/00005344-198611000-00010. [DOI] [PubMed] [Google Scholar]
- Connor H. E., Feniuk W. Role of endothelium in haemoglobin-induced contraction of dog basilar artery. Eur J Pharmacol. 1987 Aug 4;140(1):105–108. doi: 10.1016/0014-2999(87)90640-6. [DOI] [PubMed] [Google Scholar]
- Duff T. A., Louie J., Feilbach J. A., Scott G. Erythrocytes are essential for development of cerebral vasculopathy resulting from subarachnoid hemorrhage in cats. Stroke. 1988 Jan;19(1):68–72. doi: 10.1161/01.str.19.1.68. [DOI] [PubMed] [Google Scholar]
- Edwards D. H., Griffith T. M., Ryley H. C., Henderson A. H. Haptoglobin-haemoglobin complex in human plasma inhibits endothelium dependent relaxation: evidence that endothelium derived relaxing factor acts as a local autocoid. Cardiovasc Res. 1986 Aug;20(8):549–556. doi: 10.1093/cvr/20.8.549. [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Kassell N. F., Sasaki T., Nakagomi T., Lehman R. M. Selective hemoglobin inhibition of endothelium-dependent vasodilation of rabbit basilar artery. J Neurosurg. 1986 Mar;64(3):445–452. doi: 10.3171/jns.1986.64.3.0445. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Gordon J. L., Martin W. Endothelium-dependent relaxation of the pig aorta: relationship to stimulation of 86Rb efflux from isolated endothelial cells. Br J Pharmacol. 1983 Jun;79(2):531–541. doi: 10.1111/j.1476-5381.1983.tb11028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Davies R. L., Harrison T. J., Evans K. T. EDRF coordinates the behaviour of vascular resistance vessels. Nature. 1987 Oct 1;329(6138):442–445. doi: 10.1038/329442a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Davies R. L., Harrison T. J., Evans K. T. Endothelium-derived relaxing factor (EDRF) and resistance vessels in an intact vascular bed: a microangiographic study of the rabbit isolated ear. Br J Pharmacol. 1988 Mar;93(3):654–662. doi: 10.1111/j.1476-5381.1988.tb10323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Henderson A. H., Edwards D. H., Lewis M. J. Isolated perfused rabbit coronary artery and aortic strip preparations: the role of endothelium-derived relaxant factor. J Physiol. 1984 Jun;351:13–24. doi: 10.1113/jphysiol.1984.sp015228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräser T., Leisner H., Tiedt N. Absence of role of endothelium in the response of isolated porcine coronary arteries to acetylcholine. Cardiovasc Res. 1986 Apr;20(4):299–302. doi: 10.1093/cvr/20.4.299. [DOI] [PubMed] [Google Scholar]
- Holtz J., Giesler M., Bassenge E. Two dilatory mechanisms of anti-anginal drugs on epicardial coronary arteries in vivo: indirect, flow-dependent, endothelium-mediated dilation and direct smooth muscle relaxation. Z Kardiol. 1983;72 (Suppl 3):98–106. [PubMed] [Google Scholar]
- Houston D. S., Shepherd J. T., Vanhoutte P. M. Adenine nucleotides, serotonin, and endothelium-dependent relaxations to platelets. Am J Physiol. 1985 Mar;248(3 Pt 2):H389–H395. doi: 10.1152/ajpheart.1985.248.3.H389. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987 Dec;61(6):866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Ishii S., Nonaka T. [Cerebral vasospasm in subarachnoid hemorrhage--with reference to its mechanism (author's transl)]. No To Shinkei. 1977 Aug;29(8):829–840. [PubMed] [Google Scholar]
- Kalsner S. Cholinergic mechanisms in human coronary artery preparations: implications of species differences. J Physiol. 1985 Jan;358:509–526. doi: 10.1113/jphysiol.1985.sp015564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamaru K., Waga S., Kojima T., Fujimoto K., Itoh H. Endothelium-dependent relaxation of canine basilar arteries. Part 1: Difference between acetylcholine- and A23187-induced relaxation and involvement of lipoxygenase metabolite(s). Stroke. 1987 Sep-Oct;18(5):932–937. doi: 10.1161/01.str.18.5.932. [DOI] [PubMed] [Google Scholar]
- Kelm M., Feelisch M., Spahr R., Piper H. M., Noack E., Schrader J. Quantitative and kinetic characterization of nitric oxide and EDRF released from cultured endothelial cells. Biochem Biophys Res Commun. 1988 Jul 15;154(1):236–244. doi: 10.1016/0006-291x(88)90675-4. [DOI] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):529–538. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Nakagomi T., Kassell N. F., Sasaki T., Fujiwara S., Lehman R. M., Torner J. C. Impairment of endothelium-dependent vasodilation induced by acetylcholine and adenosine triphosphate following experimental subarachnoid hemorrhage. Stroke. 1987 Mar-Apr;18(2):482–489. doi: 10.1161/01.str.18.2.482. [DOI] [PubMed] [Google Scholar]
- Osaka K. Prolonged vasospasm produced by the breakdown products of erythrocytes. J Neurosurg. 1977 Sep;47(3):403–411. doi: 10.3171/jns.1977.47.3.0403. [DOI] [PubMed] [Google Scholar]
- Ozaki N., Mullan S. Possible role of the erythrocyte in causing prolonged cerebral vasospasm. J Neurosurg. 1979 Dec;51(6):773–778. doi: 10.3171/jns.1979.51.6.0773. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- TOURTELLOTTE W. W., METZ L. N., BRYAN E. R., DEJONG R. N. SPONTANEOUS SUBARACHNOID HEMORRHAGE. FACTORS AFFECTING THE RATE OF CLEARING OF THE CEREBROSPINAL FLUID. Neurology. 1964 Apr;14:301–306. doi: 10.1212/wnl.14.4.301. [DOI] [PubMed] [Google Scholar]
- Tanishima T. Cerebral vasospasm: contractile activity of hemoglobin in isolated canine basilar arteries. J Neurosurg. 1980 Dec;53(6):787–793. doi: 10.3171/jns.1980.53.6.0787. [DOI] [PubMed] [Google Scholar]
- Toda N., Ozaki T., Ohta T. Cerebrovascular sensitivity to vasoconstricting agents induced by subarachnoid hemorrhage and vasospasm in dogs. J Neurosurg. 1977 Mar;46(3):296–303. doi: 10.3171/jns.1977.46.3.0296. [DOI] [PubMed] [Google Scholar]
- Toda N., Shimizu K., Ohta T. Mechanism of cerebral arterial contraction induced by blood constituents. J Neurosurg. 1980 Sep;53(3):312–322. doi: 10.3171/jns.1980.53.3.0312. [DOI] [PubMed] [Google Scholar]
- Vermeulen M., van Gijn J., Blijenberg B. G. Spectrophotometric analysis of CSF after subarachnoid hemorrhage: limitations in the diagnosis of rebleeding. Neurology. 1983 Jan;33(1):112–115. doi: 10.1212/wnl.33.1.112. [DOI] [PubMed] [Google Scholar]
- Wellum G. R., Irvine T. W., Jr, Zervas N. T. Cerebral vasoactivity of heme proteins in vitro. Some mechanistic considerations. J Neurosurg. 1982 Jun;56(6):777–783. doi: 10.3171/jns.1982.56.6.0777. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]



