Abstract
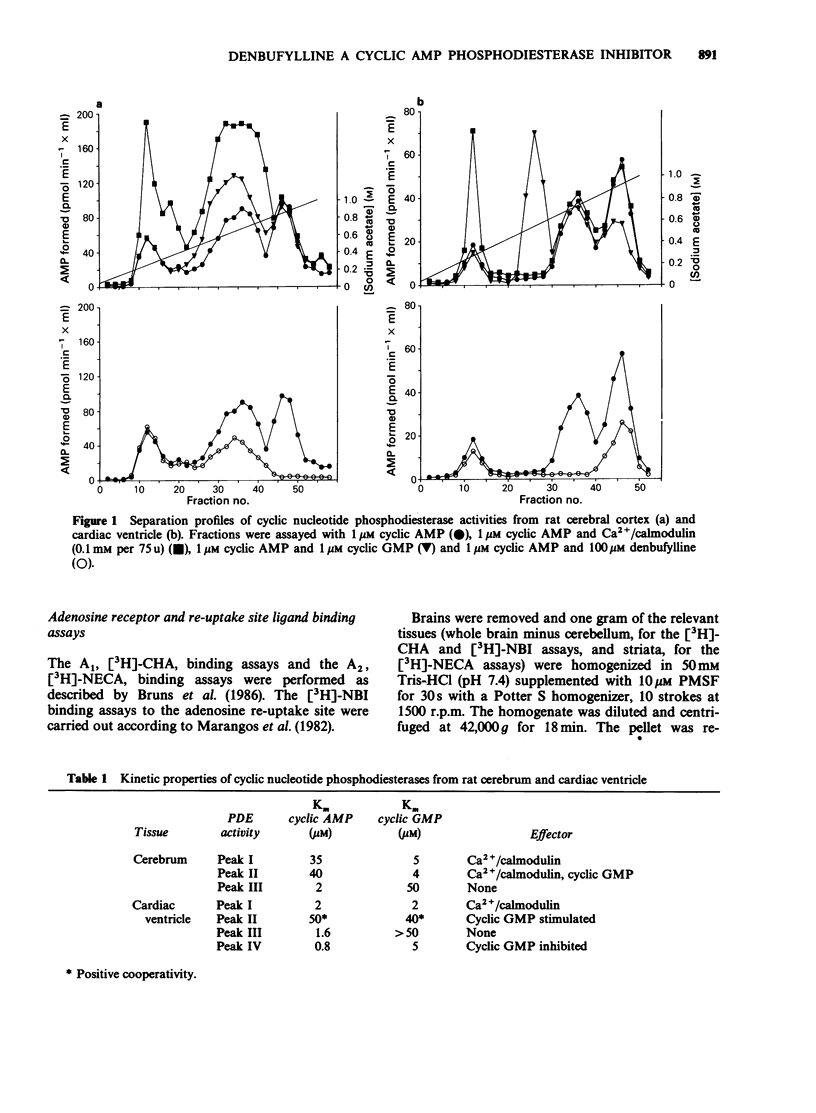
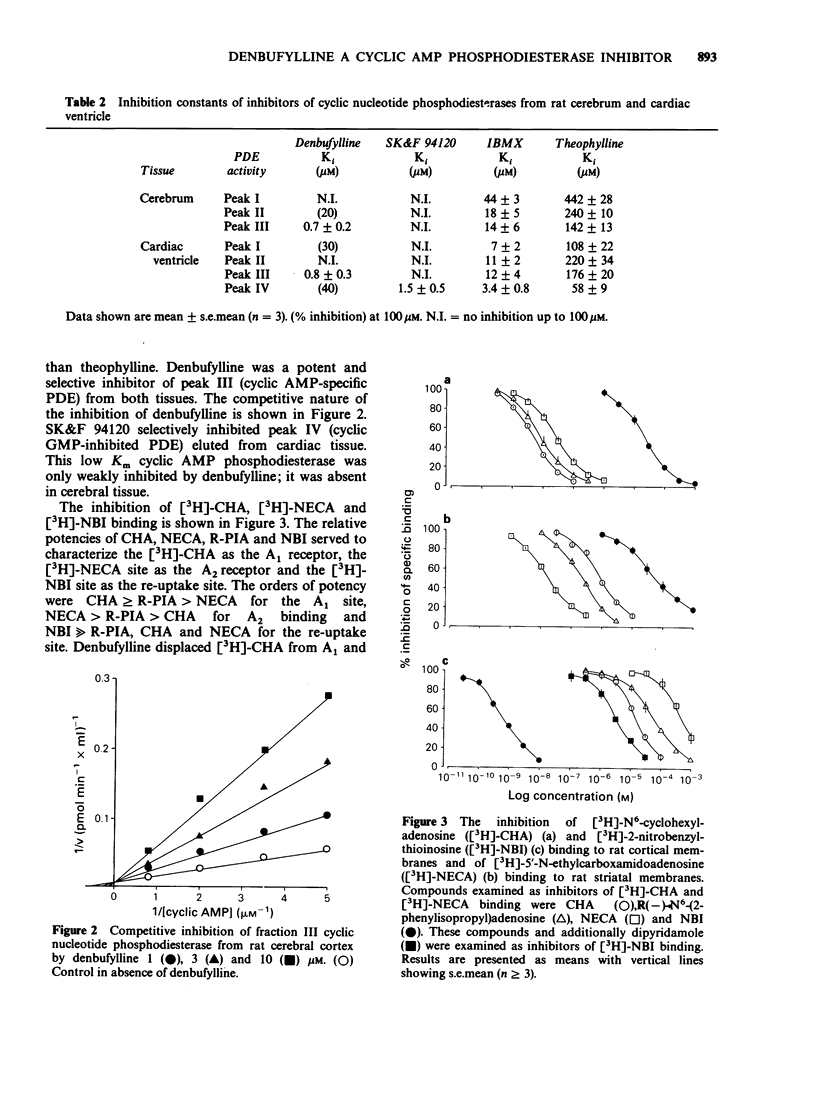
1. Denbufylline has been examined for its ability to inhibit cyclic nucleotide phosphodiesterase isoenzymes from rat cardiac ventricle and cerebrum, as well as for its affinity for adenosine A1 and A2 receptors and the re-uptake site. For comparison, SK&F 94120, theophylline and 3-isobutyl-1-methyl-xanthine (IBMX) were examined as phosphodiesterase inhibitors whilst N6-cyclohexyladenosine, R(-)-N6-(2-phenylisopropyl)-adenosine, 5'-N-ethylcarboxamido-adenosine, 2-nitrobenzylthioinosine, theophylline and IBMX were examined for their affinity for adenosine binding sites. 2. This investigation confirmed the presence of four phosphodiesterase activities in rat cardiac ventricle; in rat cerebrum only three were present. 3. Denbufylline selective inhibited one form of Ca2+-independent, low Km cyclic AMP phosphodiesterase. The form inhibited was one of two present in cardiac ventricle and the sole one in cerebrum. This form was not inhibited by cyclic GMP. The inotropic agent SK&F 94120 selectively inhibited the form of cyclic AMP phosphodiesterase which was inhibited by cyclic GMP present in cardiac ventricle. Theophylline and IBMX were relatively non-selective phosphodiesterase inhibitors. 4. Denbufylline was a less potent inhibitor of ligand binding to adenosine receptors than of cyclic AMP phosphodiesterase. This contrasted with theophylline, which had a higher affinity for adenosine receptors, and IBMX which showed no marked selectivity. Denbufylline, theophylline and IBMX all had a low affinity for the adenosine re-uptake site. 5. Denbufylline is being developed as an agent for the therapy of multi-infarct dementia. The selective inhibition of a particular low Km cyclic AMP phosphodiesterase may account for the activity of this compound.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angersbach D., Ochlich P. The effect of 7-(2'-oxopropyl)-1,3-di-n-butyl-xanthine (BRL 30892) on ischaemic skeletal muscle pO2, pH and contractility in cats and rats. Arzneimittelforschung. 1984;34(10):1274–1278. [PubMed] [Google Scholar]
- Arch J. R., Newsholme E. A. Activities and some properties of adenylate cyclase and phosphodiesterase in muscle, liver and nervous tissues from vertebrates and invertebrates in relation to the control of the concentration of adenosine 3':5'-cyclic monophosphate. Biochem J. 1976 Sep 15;158(3):603–622. doi: 10.1042/bj1580603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Bruns R. F., Lu G. H., Pugsley T. A. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986 Apr;29(4):331–346. [PubMed] [Google Scholar]
- Burnstock G., Meghji P. Distribution of P1- and P2-purinoceptors in the guinea-pig and frog heart. Br J Pharmacol. 1981 Aug;73(4):879–885. doi: 10.1111/j.1476-5381.1981.tb08741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. W. Assessment of selective inhibition of rat cerebral cortical calcium-independent and calcium-dependent phosphodiesterases in crude extracts using deoxycyclic AMP and potassium ions. Biochim Biophys Acta. 1984 Mar 1;797(3):354–362. doi: 10.1016/0304-4165(84)90257-5. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Lindström K. The xanthine derivative 1-(5'-oxohexyl)-3-methyl-7-propyl xanthine (HWA 285) enhances the actions of adenosine. Acta Pharmacol Toxicol (Copenh) 1986 Mar;58(3):187–192. doi: 10.1111/j.1600-0773.1986.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Persson C. G. Xanthine derivatives as adenosine receptor antagonists. Eur J Pharmacol. 1982 Jul 30;81(4):673–676. doi: 10.1016/0014-2999(82)90359-4. [DOI] [PubMed] [Google Scholar]
- Goelet P., Castellucci V. F., Schacher S., Kandel E. R. The long and the short of long-term memory--a molecular framework. 1986 Jul 31-Aug 6Nature. 322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Gray R., Johnston D. Noradrenaline and beta-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature. 1987 Jun 18;327(6123):620–622. doi: 10.1038/327620a0. [DOI] [PubMed] [Google Scholar]
- Gristwood R. W., Eden R. J., Owen D. A., Taylor E. M. Pharmacological studies with SK&F 94120, a novel positive inotropic agent with vasodilator activity. J Pharm Pharmacol. 1986 Jun;38(6):452–459. doi: 10.1111/j.2042-7158.1986.tb04609.x. [DOI] [PubMed] [Google Scholar]
- Gristwood R. W., English T. A., Wallwork J., Sampford K. A., Owen D. A. Analysis of responses to a selective phosphodiesterase III inhibitor, SK&F 94120, on isolated myocardium, including human ventricular myocardium from "end-stage" failure patients. J Cardiovasc Pharmacol. 1987 Jun;9(6):719–727. doi: 10.1097/00005344-198706000-00013. [DOI] [PubMed] [Google Scholar]
- Grome J. J., Stefanovich V. Differential effects of methylxanthines on local cerebral blood flow and glucose utilization in the conscious rat. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jun;333(2):172–177. doi: 10.1007/BF00506522. [DOI] [PubMed] [Google Scholar]
- Jarrott D. M., Domer F. R. A gerbil model of cerebral ischemia suitable for drug evaluation. Stroke. 1980 Mar-Apr;11(2):203–209. doi: 10.1161/01.str.11.2.203. [DOI] [PubMed] [Google Scholar]
- Jukna J. J., Nicholson C. D. The effect of denbufylline on the viscosity of rat whole blood and on the deformability (filterability) of rat blood cell suspensions. Naunyn Schmiedebergs Arch Pharmacol. 1987 Apr;335(4):445–448. doi: 10.1007/BF00165561. [DOI] [PubMed] [Google Scholar]
- Kincaid R. L., Manganiello V. C., Odya C. E., Osborne J. C., Jr, Stith-Coleman I. E., Danello M. A., Vaughan M. Purification and properties of calmodulin-stimulated phosphodiesterase from mammalian brain. J Biol Chem. 1984 Apr 25;259(8):5158–5166. [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Schlegel W., Rodbell M. Adenosine analogs inhibit adipocyte adenylate cyclase by a GTP-dependent process: basis for actions of adenosine and methylxanthines on cyclic AMP production and lipolysis. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5362–5366. doi: 10.1073/pnas.75.11.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugnier C., Schoeffter P., Le Bec A., Strouthou E., Stoclet J. C. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem Pharmacol. 1986 May 15;35(10):1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- Marangos P. J., Patel J., Clark-Rosenberg R., Martino A. M. [3H]nitrobenzylthioinosine binding as a probe for the study of adenosine uptake sites in brain. J Neurochem. 1982 Jul;39(1):184–191. doi: 10.1111/j.1471-4159.1982.tb04717.x. [DOI] [PubMed] [Google Scholar]
- Mathew R. J., Barr D. L., Weinman M. L. Caffeine and cerebral blood flow. Br J Psychiatry. 1983 Dec;143:604–608. doi: 10.1192/bjp.143.6.604. [DOI] [PubMed] [Google Scholar]
- Reeves M. L., Leigh B. K., England P. J. The identification of a new cyclic nucleotide phosphodiesterase activity in human and guinea-pig cardiac ventricle. Implications for the mechanism of action of selective phosphodiesterase inhibitors. Biochem J. 1987 Jan 15;241(2):535–541. doi: 10.1042/bj2410535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolphi K. A., Keil M., Hinze H. J. Effect of theophylline on ischemically induced hippocampal damage in Mongolian gerbils: a behavioral and histopathological study. J Cereb Blood Flow Metab. 1987 Feb;7(1):74–81. doi: 10.1038/jcbfm.1987.11. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem. 1958 Jun;232(2):1077–1091. [PubMed] [Google Scholar]
- Schwabe U., Ukena D., Lohse M. J. Xanthine derivatives as antagonists at A1 and A2 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1985 Sep;330(3):212–221. doi: 10.1007/BF00572436. [DOI] [PubMed] [Google Scholar]
- Smellie F. W., Davis C. W., Daly J. W., Wells J. N. Alkylxanthines: inhibition of adenosine-elicited accumulation of cyclic AMP in brain slices and of brain phosphodiesterase activity. Life Sci. 1979 Jun 25;24(26):2475–2482. doi: 10.1016/0024-3205(79)90458-2. [DOI] [PubMed] [Google Scholar]
- Stefanovich V. Uptake of adenosine by isolated bovine cortex microvessels. Neurochem Res. 1983 Nov;8(11):1459–1469. doi: 10.1007/BF00965001. [DOI] [PubMed] [Google Scholar]
- Strada S. J., Martin M. W., Thompson W. J. General properties of multiple molecular forms of cyclic nucleotide phosphodiesterase in the nervous system. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:13–29. [PubMed] [Google Scholar]
- THER L., MUSCHAWECK R., HERGOTT J. Antagonismus zwischen Adenosin und Methyl-Xanthinen am Reizleitungssystem des Herzens. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1957;231(6):586–590. [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Characterization of cyclic nucleotide phosphodiesterases of rat tissues. J Biol Chem. 1971 May 25;246(10):3145–3150. [PubMed] [Google Scholar]
- Weishaar R. E., Burrows S. D., Kobylarz D. C., Quade M. M., Evans D. B. Multiple molecular forms of cyclic nucleotide phosphodiesterase in cardiac and smooth muscle and in platelets. Isolation, characterization, and effects of various reference phosphodiesterase inhibitors and cardiotonic agents. Biochem Pharmacol. 1986 Mar 1;35(5):787–800. doi: 10.1016/0006-2952(86)90247-9. [DOI] [PubMed] [Google Scholar]
- Weishaar R. E., Cain M. H., Bristol J. A. A new generation of phosphodiesterase inhibitors: multiple molecular forms of phosphodiesterase and the potential for drug selectivity. J Med Chem. 1985 May;28(5):537–545. doi: 10.1021/jm50001a001. [DOI] [PubMed] [Google Scholar]
- Wu P. H., Phillis J. W., Nye M. J. Alkylxanthines as adenosine receptor antagonists and membrane phosphodiesterase inhibitors in central nervous tissue: evaluation of structure-activity relationships. Life Sci. 1982 Dec 20;31(25):2857–2867. doi: 10.1016/0024-3205(82)90676-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Lieberman F., Osborne J. C., Jr, Manganiello V. C., Vaughan M., Hidaka H. Selective inhibition of two soluble adenosine cyclic 3',5'-phosphate phosphodiesterases partially purified from calf liver. Biochemistry. 1984 Feb 14;23(4):670–675. doi: 10.1021/bi00299a013. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


