Abstract
1. The effects of buprenorphine, given intravenously, on the incidence and severity of early acute coronary artery occlusion-induced arrhythmias were examined in anaesthetised rats. The electrophysiological effects of buprenorphine were also examined in sheep Purkinje fibres and rat papillary muscles, superfused in vitro with either a normal or a hypoxic, hyperkalaemic and acidotic physiological salt solution (PSS). 2. In anaesthetised rats subjected to acute coronary artery occlusion, pretreatment with buprenorphine (1 mg kg-1 i.v.) markedly reduced the incidence of ventricular extra-systoles during the initial 30 min post-occlusion period. The incidence of ventricular fibrillation (VF) was also significantly reduced from 56% to 10%. 3. At the antiarrhythmic dose (1 mg kg -1), buprenorphine also attenuated the sudden fall in systemic arterial blood pressure induced by acute coronary artery ligation. 4. In normal sheep Purkinje fibres and rat papillary muscles, buprenorphine (10(-6)-10(-5) M) significantly reduced the action potential height and maximum rate of depolarisation of phase zero (MRD) and prolonged the duration of the action potential. 5. Superfusion of sheep Purkinje fibres and rat papillary muscles with a hypoxic, hyperkalaemic and acidotic PSS resulted in marked reductions in resting membrane potential, upstroke and duration of the action potential. 6. In the presence of the modified compared with normal PSS, buprenorphine reduced the action potential height and MRD of both sheep Purkinje fibres and rat papillary muscles to a greater extent, although its ability to prolong the action potential duration was attenuated. 7. The antiarrhythmic effects of buprenorphine observed in vivo may be explained by its direct cardiac electrophysiological effects.(ABSTRACT TRUNCATED AT 250 WORDS)
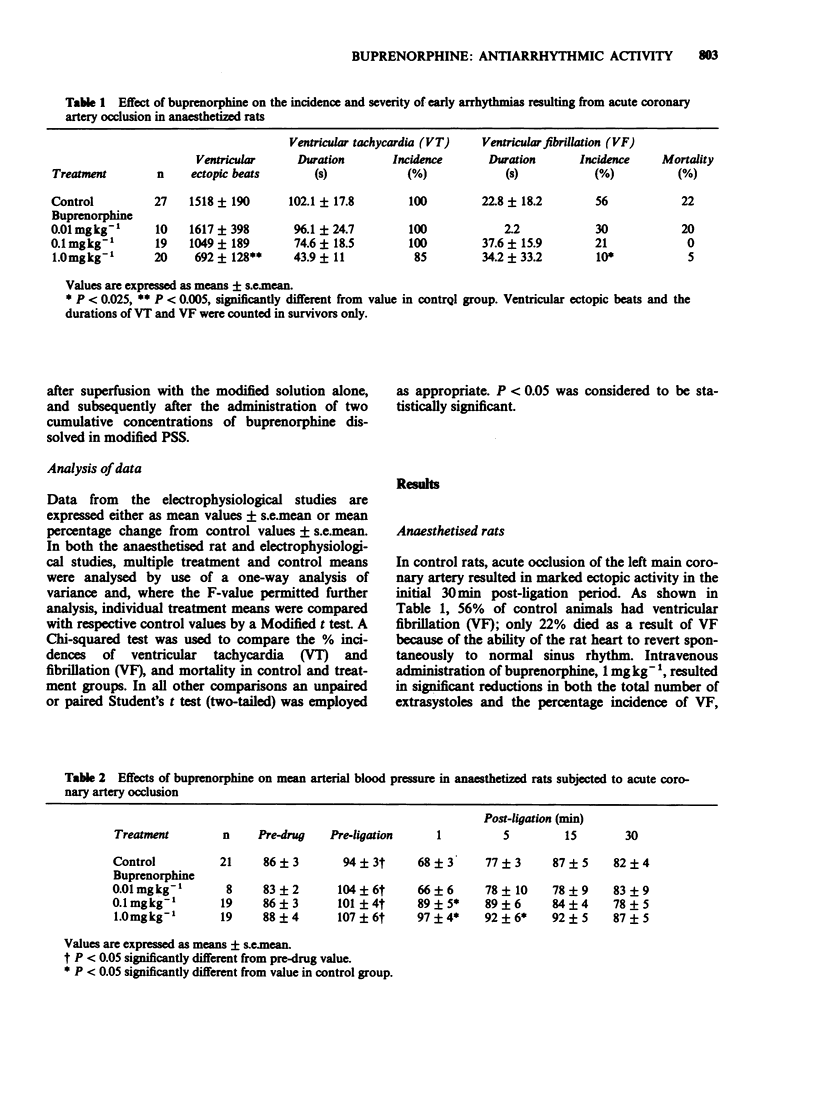
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boachie-Ansah G., Kane K. A., Parratt J. R. Cardiac electrophysiological effects of isoprenaline, phenylephrine, and noradrenaline on normal and mildly "ischaemic" sheep Purkinje fibers. J Cardiovasc Pharmacol. 1989 Feb;13(2):291–298. doi: 10.1097/00005344-198902000-00018. [DOI] [PubMed] [Google Scholar]
- Botting J. H., Curtis M. J., Walker M. J. Arrhythmias associated with myocardial ischaemia and infarction. Mol Aspects Med. 1985;8(4):307–422. doi: 10.1016/0098-2997(85)90014-7. [DOI] [PubMed] [Google Scholar]
- Brasch H. Influence of the optical isomers (+)- and (-)-naloxone on beating frequency, contractile force and action potentials of guinea-pig isolated cardiac preparations. Br J Pharmacol. 1986 Aug;88(4):733–740. doi: 10.1111/j.1476-5381.1986.tb16245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. Selectivity of antiarrhythmic drugs and ionic channels: a historical overview. Ann N Y Acad Sci. 1984;427:1–15. doi: 10.1111/j.1749-6632.1984.tb20771.x. [DOI] [PubMed] [Google Scholar]
- Clark C., Foreman M. I., Kane K. A., McDonald F. M., Parratt J. R. Coronary artery ligation in anesthetized rats as a method for the production of experimental dysrhythmias and for the determination of infarct size. J Pharmacol Methods. 1980 Jun;3(4):357–368. doi: 10.1016/0160-5402(80)90077-7. [DOI] [PubMed] [Google Scholar]
- Cohen M. R., Cohen R. M., Pickar D., Murphy D. L., Bunney W. E., Jr Physiological effects of high dose naloxone administration to normal adults. Life Sci. 1982 Jun 7;30(23):2025–2031. doi: 10.1016/0024-3205(82)90443-x. [DOI] [PubMed] [Google Scholar]
- Downar E., Janse M. J., Durrer D. The effect of acute coronary artery occlusion on subepicardial transmembrane potentials in the intact porcine heart. Circulation. 1977 Aug;56(2):217–224. doi: 10.1161/01.cir.56.2.217. [DOI] [PubMed] [Google Scholar]
- Evans J. J., Gilmour R. F., Jr, Zipes D. P. The effects of lidocaine and quinidine on impulse propagation across the canine Purkinje-muscle junction during combined hyperkalemia, hypoxia, and acidosis. Circ Res. 1984 Aug;55(2):185–196. doi: 10.1161/01.res.55.2.185. [DOI] [PubMed] [Google Scholar]
- Fagbemi O., Kane K. A., Leprán I., Parratt J. R., Szekeres L. Antiarrhythmic actions of meptazinol, a partial agonist at opiate receptors, in acute myocardial ischaemia. Br J Pharmacol. 1983 Mar;78(3):455–460. doi: 10.1111/j.1476-5381.1983.tb08805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagbemi O., Leprán I., Parratt J. R., Szekeres L. Naloxone inhibits early arrhythmias resulting from acute coronary ligation. Br J Pharmacol. 1982 Aug;76(4):504–506. doi: 10.1111/j.1476-5381.1982.tb09246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour R. F., Jr, Zipes D. P. Different electrophysiological responses of canine endocardium and epicardium to combined hyperkalemia, hypoxia, and acidosis. Circ Res. 1980 Jun;46(6):814–825. doi: 10.1161/01.res.46.6.814. [DOI] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Antiarrhythmic agents: the modulated receptor mechanism of action of sodium and calcium channel-blocking drugs. Annu Rev Pharmacol Toxicol. 1984;24:387–423. doi: 10.1146/annurev.pa.24.040184.002131. [DOI] [PubMed] [Google Scholar]
- Huang X. D., Lee A. Y., Wong T. M., Zhan C. Y., Zhao Y. Y. Naloxone inhibits arrhythmias induced by coronary artery occlusion and reperfusion in anaesthetized dogs. Br J Pharmacol. 1986 Mar;87(3):475–477. doi: 10.1111/j.1476-5381.1986.tb10186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parratt J. R., Sitsapesan R. Stereospecific antiarrhythmic effect of opioid receptor antagonists in myocardial ischaemia. Br J Pharmacol. 1986 Apr;87(4):621–622. doi: 10.1111/j.1476-5381.1986.tb14577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadée W., Richards M. L., Grevel J., Rosenbaum J. S. In vivo characterization of four types of opioid binding sites in rat brain. Life Sci. 1983;33 (Suppl 1):187–189. doi: 10.1016/0024-3205(83)90474-5. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R., Parratt J. R. The effects of drugs interacting with opioid receptors on the early ventricular arrhythmias arising from myocardial ischaemia. Br J Pharmacol. 1989 Jul;97(3):795–800. doi: 10.1111/j.1476-5381.1989.tb12018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori A. E., Ikeda M., Portoghese P. S. The mu, kappa and delta properties of various opioid agonists. Eur J Pharmacol. 1986 Apr 29;123(3):357–361. doi: 10.1016/0014-2999(86)90709-0. [DOI] [PubMed] [Google Scholar]



