Abstract
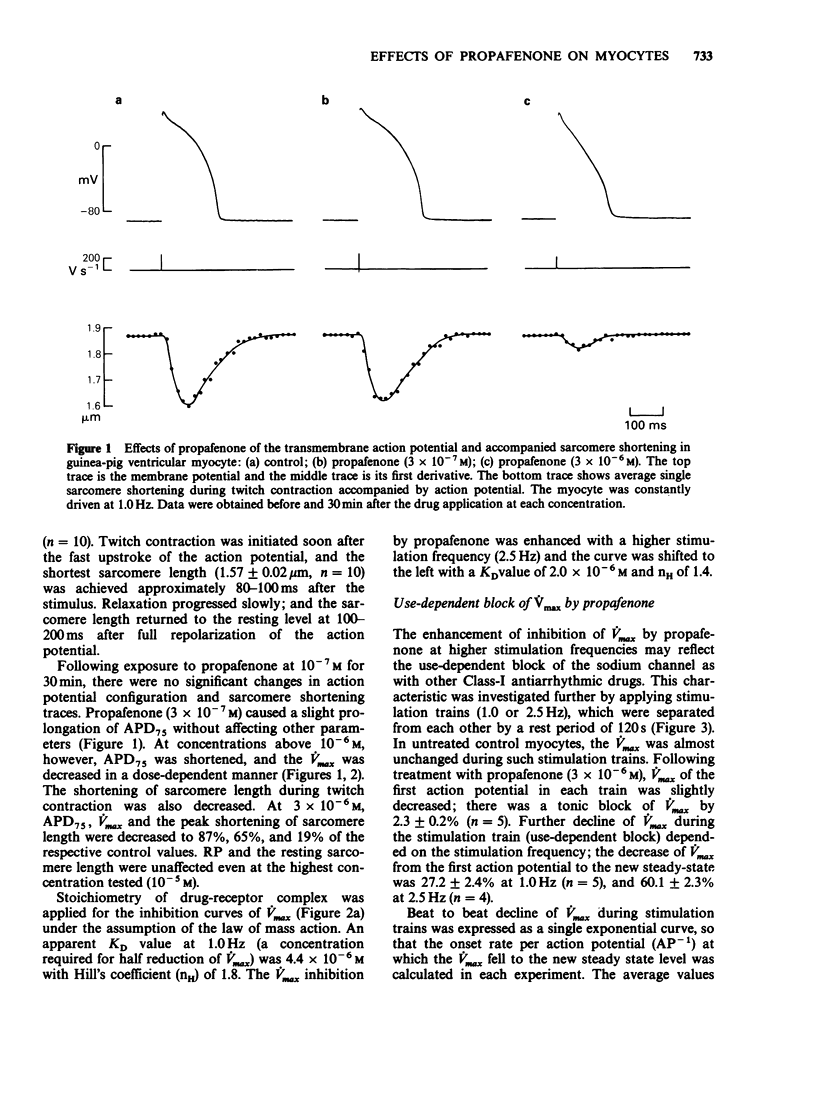
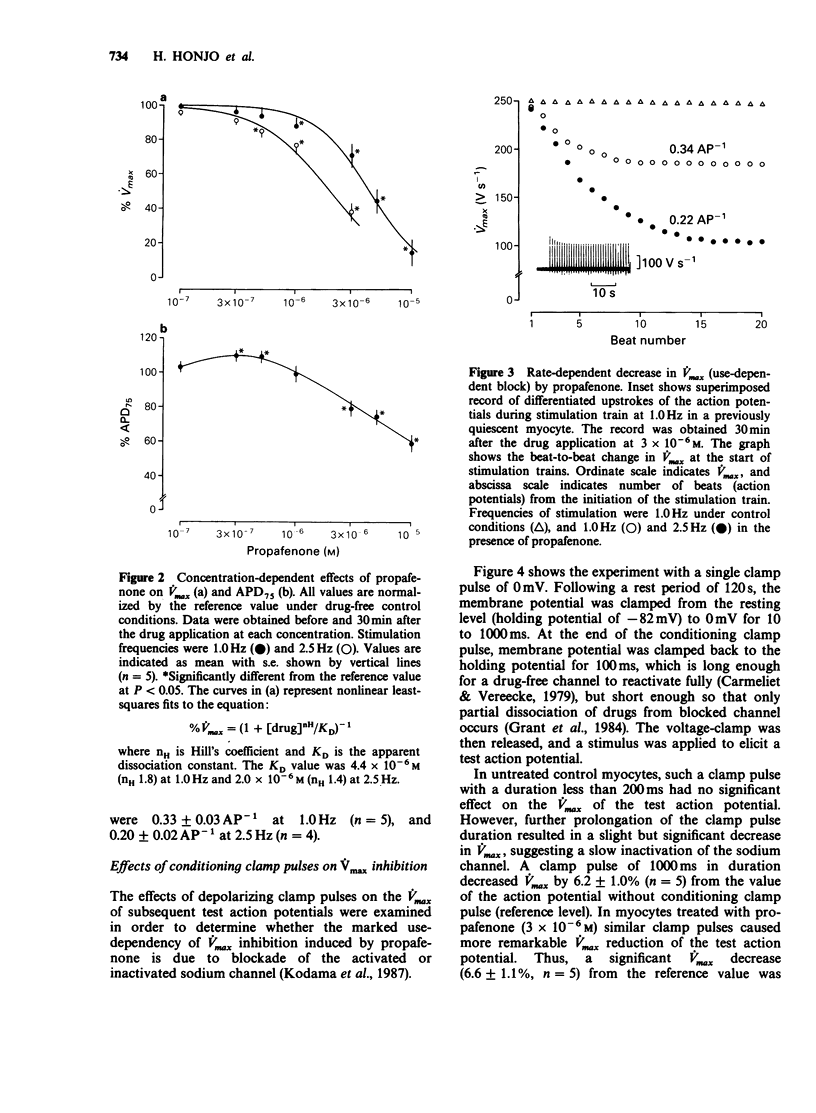
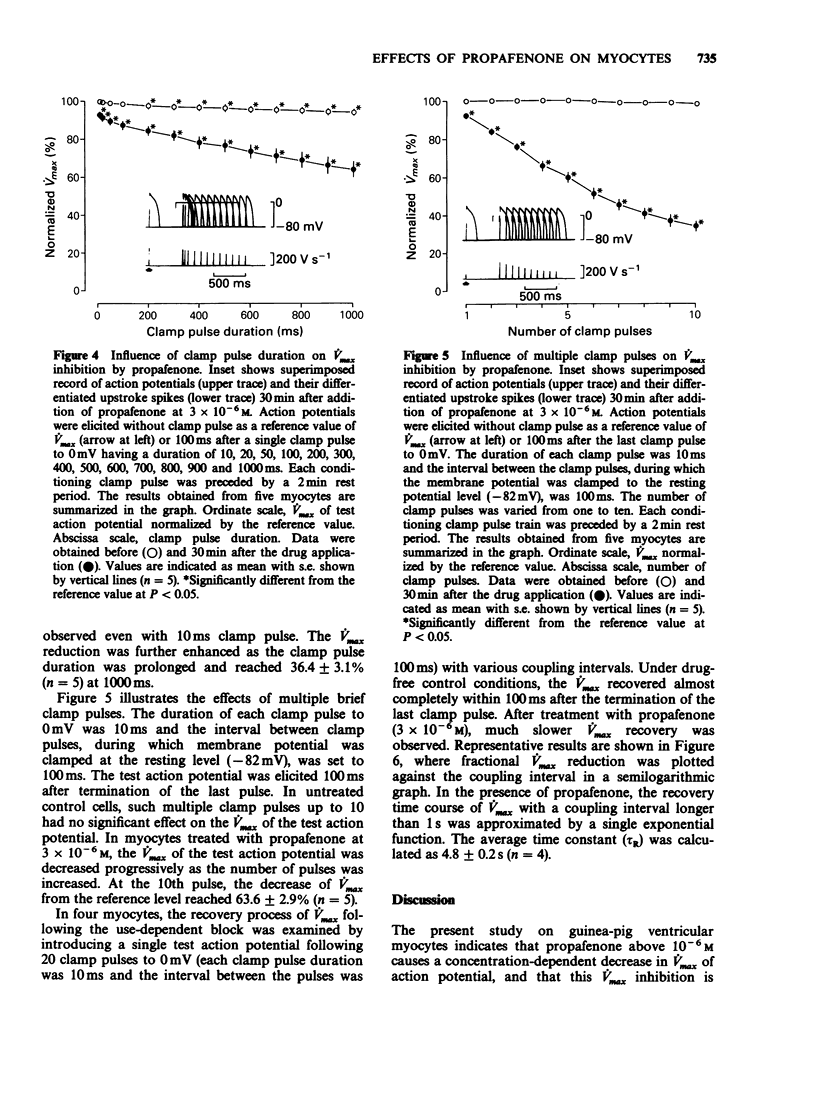
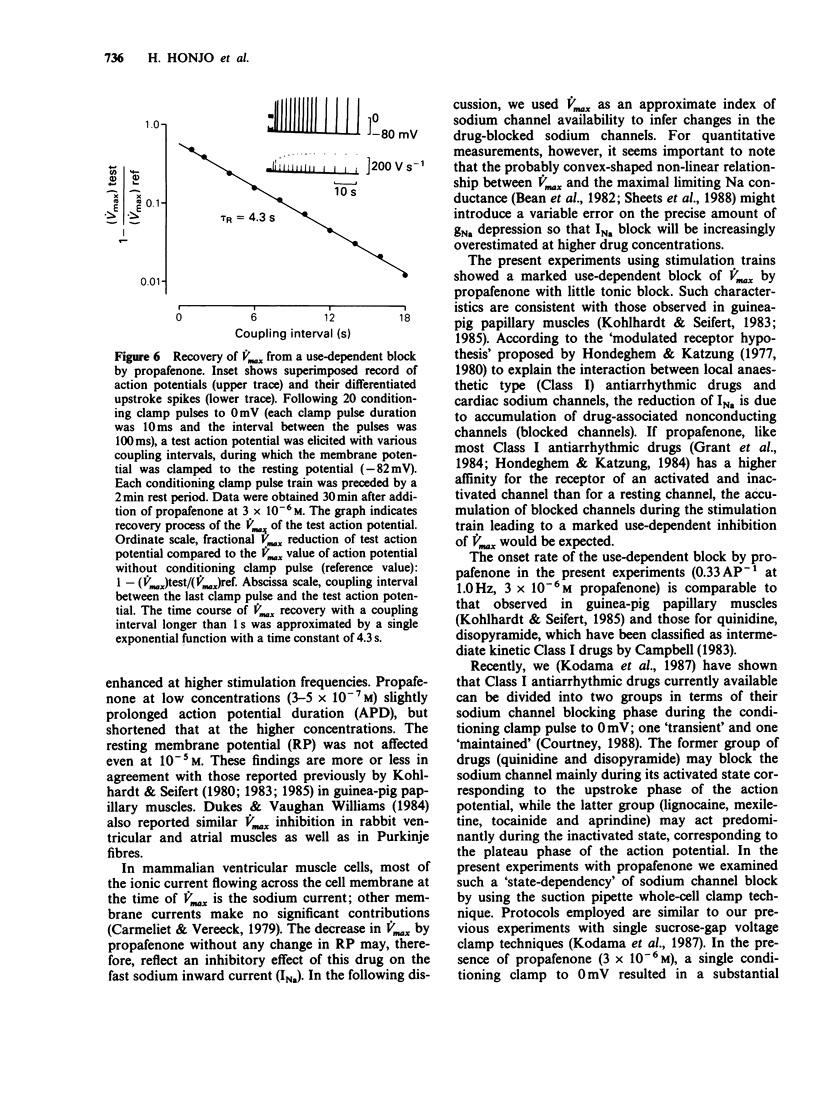
1. The effects of propafenone on the transmembrane action potential and sarcomere shortening during twitch contraction were investigated in single ventricular myocytes isolated from guinea-pig hearts. 2. Propafenone at low concentrations (3-5 x 10(-7) M) slightly lengthened action potential duration (APD), but shortened it at higher concentrations. The shortening of APD was accompanied by an attenuation of sarcomere shortening during twitch contraction. 3. Propafenone (greater than 10(-6) M) caused a concentration-dependent decrease in the maximum upstroke velocity (Vmax) of the action potential. In the presence of propafenone (3 x 10(-6) M), trains of stimuli led to an exponential decline in Vmax. A time constant for the recovery of Vmax from the use-dependent block was 4.8 s. 4. In myocytes treated with propafenone (3 x 10(-6) M), the Vmax of test action potentials preceded by the conditioning clamp pulses to 0 mV was progressively decreased by increasing the duration of single clamp pulse or by increasing the number of multiple brief clamp pulses. 5. These findings suggest that propafenone has use-dependent inhibitory action on the sodium channel by binding to the channel during both activated and inactivated states, and that the unbinding rate is comparable to that of Class-I antiarrhythmic drugs with intermediate kinetics. Propafenone may also have an inhibitory action on calcium and potassium channels.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell T. J. Kinetics of onset of rate-dependent effects of Class I antiarrhythmic drugs are important in determining their effects on refractoriness in guinea-pig ventricle, and provide a theoretical basis for their subclassification. Cardiovasc Res. 1983 Jun;17(6):344–352. doi: 10.1093/cvr/17.6.344. [DOI] [PubMed] [Google Scholar]
- Clarkson C. W., Matsubara T., Hondeghem L. M. Slow inactivation of Vmax in guinea pig ventricular myocardium. Am J Physiol. 1984 Oct;247(4 Pt 2):H645–H654. doi: 10.1152/ajpheart.1984.247.4.H645. [DOI] [PubMed] [Google Scholar]
- Connolly S. J., Kates R. E., Lebsack C. S., Echt D. S., Mason J. W., Winkle R. A. Clinical efficacy and electrophysiology of oral propafenone for ventricular tachycardia. Am J Cardiol. 1983 Dec 1;52(10):1208–1213. doi: 10.1016/0002-9149(83)90575-1. [DOI] [PubMed] [Google Scholar]
- Courtney K. R. Why do some drugs preferentially block open sodium channels? J Mol Cell Cardiol. 1988 Jun;20(6):461–464. doi: 10.1016/s0022-2828(88)80073-7. [DOI] [PubMed] [Google Scholar]
- Dukes I. D., Vaughan Williams E. M. The multiple modes of action of propafenone. Eur Heart J. 1984 Feb;5(2):115–125. doi: 10.1093/oxfordjournals.eurheartj.a061621. [DOI] [PubMed] [Google Scholar]
- Grant A. O., Starmer C. F., Strauss H. C. Antiarrhythmic drug action. Blockade of the inward sodium current. Circ Res. 1984 Oct;55(4):427–439. doi: 10.1161/01.res.55.4.427. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Antiarrhythmic agents: the modulated receptor mechanism of action of sodium and calcium channel-blocking drugs. Annu Rev Pharmacol Toxicol. 1984;24:387–423. doi: 10.1146/annurev.pa.24.040184.002131. [DOI] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Hondeghem L., Katzung B. G. Test of a model of antiarrhythmic drug action. Effects of quinidine and lidocaine on myocardial conduction. Circulation. 1980 Jun;61(6):1217–1224. doi: 10.1161/01.cir.61.6.1217. [DOI] [PubMed] [Google Scholar]
- Kodama I., Toyama J., Takanaka C., Yamada K. Block of activated and inactivated sodium channels by class-I antiarrhythmic drugs studied by using the maximum upstroke velocity (Vmax) of action potential in guinea-pig cardiac muscles. J Mol Cell Cardiol. 1987 Apr;19(4):367–377. doi: 10.1016/s0022-2828(87)80582-5. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Seifert C., Hondeghem L. M. Tonic and phasic INa blockade by antiarrhythmics. Different properties of drug binding to fast sodium channels as judged from Vmax studies with propafenone and derivatives in mammalian ventricular myocardium. Pflugers Arch. 1983 Mar 1;396(3):199–209. doi: 10.1007/BF00587856. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Seifert C. Inhibition of Vmax of the action potential by propafenone and its voltage-, time- and pH-dependence in mammalian ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1980;315(1):55–62. doi: 10.1007/BF00504230. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Seifert C. Properties of Vmax block of INa-mediated action potentials during combined application of antiarrhythmic drugs in cardiac muscle. Naunyn Schmiedebergs Arch Pharmacol. 1985 Sep;330(3):235–244. doi: 10.1007/BF00572439. [DOI] [PubMed] [Google Scholar]
- Ledda F., Mantelli L., Manzini S., Amerini S., Mugelli A. Electrophysiological and antiarrhythmic properties of propafenon in isolated cardiac preparations. J Cardiovasc Pharmacol. 1981 Nov-Dec;3(6):1162–1173. doi: 10.1097/00005344-198111000-00002. [DOI] [PubMed] [Google Scholar]
- Sato T., Watanabe T., Honjo H., Naito Y., Kodama I., Toyama J. Microcomputer-based image processing system for measuring sarcomere motion of single cardiac cells. IEEE Trans Biomed Eng. 1988 May;35(5):397–400. doi: 10.1109/10.1400. [DOI] [PubMed] [Google Scholar]
- Satoh H., Hashimoto K. Effect of propafenone on the membrane currents of rabbit sino-atrial node cells. Eur J Pharmacol. 1984 Mar 23;99(2-3):185–191. doi: 10.1016/0014-2999(84)90240-1. [DOI] [PubMed] [Google Scholar]
- Seipel L., Breithardt G. Propafenone--a new antiarrhythmic drug. Eur Heart J. 1980 Aug;1(4):309–313. doi: 10.1093/oxfordjournals.eurheartj.a061135. [DOI] [PubMed] [Google Scholar]
- Sheets M. F., Hanck D. A., Fozzard H. A. Nonlinear relation between Vmax and INa in canine cardiac Purkinje cells. Circ Res. 1988 Aug;63(2):386–398. doi: 10.1161/01.res.63.2.386. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Rautaharju P. M., McDonald T. F. Ventricular action potentials, ventricular extracellular potentials, and the ECG of guinea pig. Circ Res. 1985 Sep;57(3):362–373. doi: 10.1161/01.res.57.3.362. [DOI] [PubMed] [Google Scholar]


