Abstract
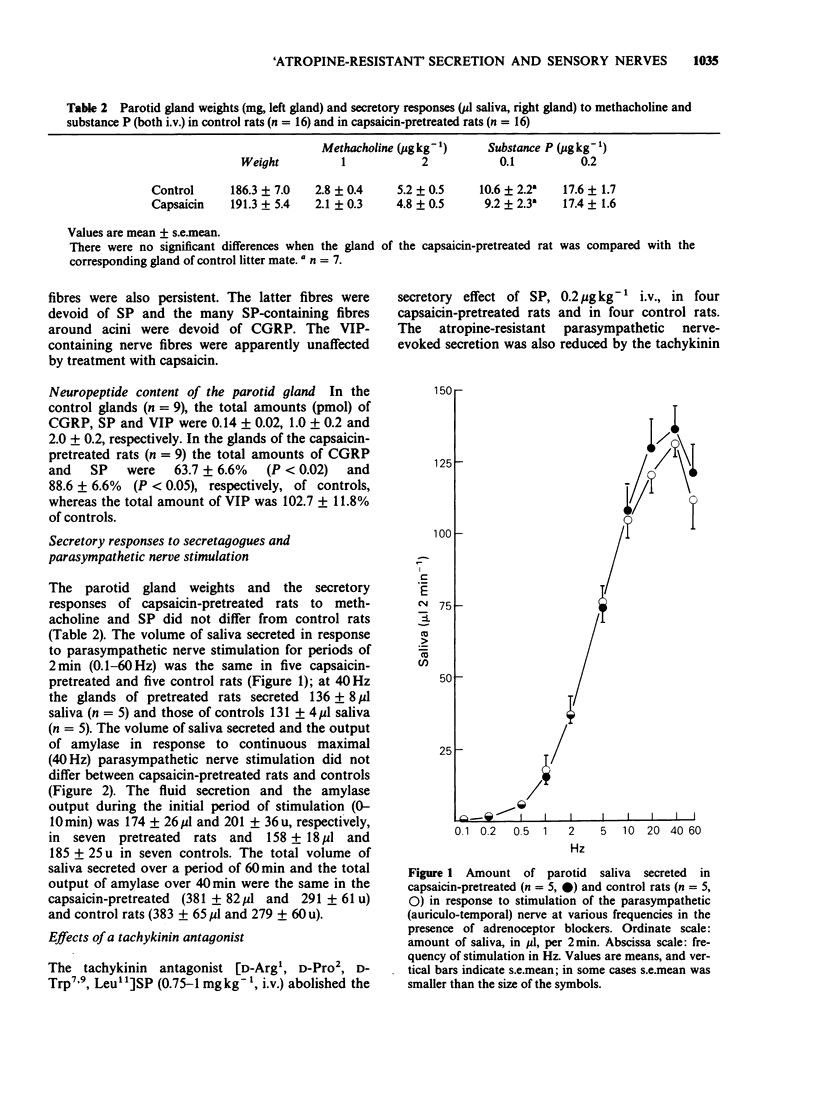
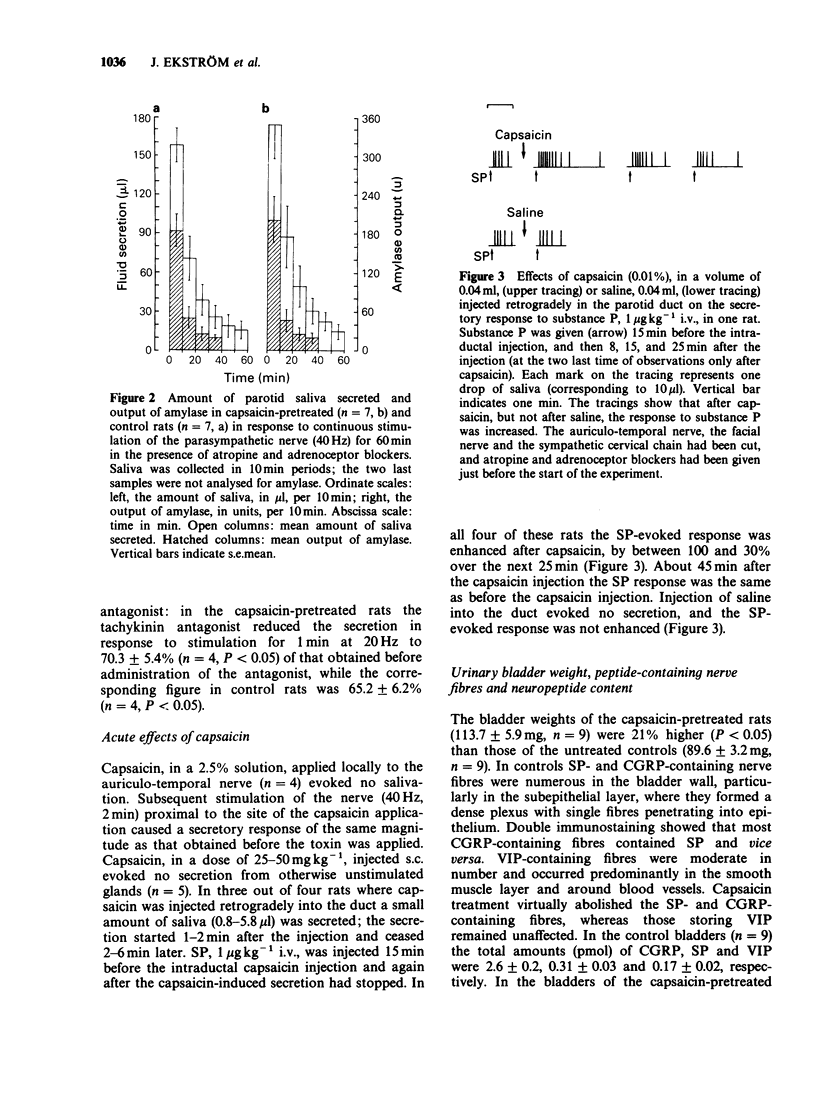
1. 'Atropine-resistant' secretion of saliva in response to parasympathetic stimulation may reflect antidromic activation of sensory nerve fibres. In this investigation, the effect of pretreatment in the rat with capsaicin (total dose of 125 mg kg-1, s.c.), was determined. 2. In the parotid glands substance P (SP)/calcitonin gene-related peptide (CGRP)-containing nerve fibres around ducts and blood vessels disappeared after capsaicin, while periacinar SP-containing fibres (devoid of CGRP) and CGRP-containing fibres (devoid of SP) remained. Vasoactive intestinal peptide (VIP)-containing nerve fibres seemed to be unaffected. The parotid content of SP and CGRP was reduced by 11 and 36% respectively, while that of VIP remained unchanged. 3. The weights of the parotid glands and their sensitivity to the secretagogues methacholine and SP, injected intravenously, were unchanged as was the response to stimulation of the auriculo-temporal nerve in the presence and absence of atropine. 4. In contrast to capsaicin pretreatment, parasympathetic denervation of the parotid gland reduced the weight of the gland and produced an increase in the response to methacholine and SP. 5. For comparison, the effectiveness of the capsaicin treatment on neuropeptide content was determined in the urinary bladder. The bladder of capsaicin-pretreated rats increased in weight (21%) and in VIP content (31%), while the content of SP and CGRP was reduced by 86 and 94%, respectively. SP- and CGRP-containing nerve fibres were virtually eliminated, while VIP-containing nerve fibres seemed unaffected. 6. In conclusion, antidromic activation of primary afferent (capsaicin-sensitive) C-fibres does not contribute significantly to the 'atropine-resistant' secretory response of the parotid gland to stimulation of the parasympathetic nerve.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alm P., Ekström J. Cholinergic nerves of unknown origin in the parotid glands of rats. Arch Oral Biol. 1976;21(7):417–421. doi: 10.1016/0003-9969(76)90005-4. [DOI] [PubMed] [Google Scholar]
- Brodin E., Lindefors N., Dalsgaard C. J., Theodorsson-Norheim E., Rosell S. Tachykinin multiplicity in rat central nervous system as studied using antisera raised against substance P and neurokinin A. Regul Pept. 1986 Feb;13(3-4):253–272. doi: 10.1016/0167-0115(86)90044-3. [DOI] [PubMed] [Google Scholar]
- Buck S. H., Burks T. F. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986 Sep;38(3):179–226. [PubMed] [Google Scholar]
- Ekström J., Brodin E., Ekman R., Håkanson R., Månsson B., Tobin G. Depletion of neuropeptides in rat parotid glands and declining atropine-resistant salivary secretion upon continuous parasympathetic nerve stimulation. Regul Pept. 1985 Aug;11(4):353–359. doi: 10.1016/0167-0115(85)90207-1. [DOI] [PubMed] [Google Scholar]
- Ekström J., Brodin E., Ekman R., Håkanson R., Sundler F. Vasoactive intestinal peptide and substance P in salivary glands of the rat following denervation or duct ligation. Regul Pept. 1984 Dec;10(1):1–10. doi: 10.1016/0167-0115(84)90047-8. [DOI] [PubMed] [Google Scholar]
- Ekström J. Choline acetyltransferase activity in rat salivary glands after cellulose rich diet or treatment with an atropine-like drug. Q J Exp Physiol Cogn Med Sci. 1974 Jul;59(3):191–199. doi: 10.1113/expphysiol.1974.sp002261. [DOI] [PubMed] [Google Scholar]
- Ekström J., Ekman R., Håkanson R., Sjögren S., Sundler F. Calcitonin gene-related peptide in rat salivary glands: neuronal localization, depletion upon nerve stimulation, and effects on salivation in relation to substance P. Neuroscience. 1988 Sep;26(3):933–949. doi: 10.1016/0306-4522(88)90110-8. [DOI] [PubMed] [Google Scholar]
- Ekström J., Månsson B., Tobin G., Garrett J. R., Thulin A. Atropine-resistant secretion of parotid saliva on stimulation of the auriculo-temporal nerve. Acta Physiol Scand. 1983 Dec;119(4):445–449. doi: 10.1111/j.1748-1716.1983.tb07360.x. [DOI] [PubMed] [Google Scholar]
- Ekström J., Månsson B., Tobin G. Vasoactive intestinal peptide evoked secretion of fluid and protein from rat salivary glands and the development of supersensitivity. Acta Physiol Scand. 1983;119(2):169–175. doi: 10.1111/j.1748-1716.1983.tb07322.x. [DOI] [PubMed] [Google Scholar]
- Ekström J. Neuropeptides and secretion. J Dent Res. 1987 Feb;66(2):524–530. doi: 10.1177/00220345870660022301. [DOI] [PubMed] [Google Scholar]
- Ekström J., Olgart L. Complementary action of substance P and vasoactive intestinal peptide on the rat parotid secretion. Acta Physiol Scand. 1986 Jan;126(1):25–31. doi: 10.1111/j.1748-1716.1986.tb07784.x. [DOI] [PubMed] [Google Scholar]
- Ekström J. Sensitization of the rat parotid gland to secretagogues following either parasympathetic denervation or sympathetic denervation or decentralization. Acta Physiol Scand. 1980 Mar;108(3):253–261. doi: 10.1111/j.1748-1716.1980.tb06531.x. [DOI] [PubMed] [Google Scholar]
- Ekström J., Wahlestedt C. Supersensitivity to substance P and physalaemin in rat salivary glands after denervation or decentralization. Acta Physiol Scand. 1982 Aug;115(4):437–446. doi: 10.1111/j.1748-1716.1982.tb07102.x. [DOI] [PubMed] [Google Scholar]
- Grunditz T., Ekman R., Håkanson R., Rerup C., Sundler F., Uddman R. Calcitonin gene-related peptide in thyroid nerve fibers and C cells: effects on thyroid hormone secretion and response to hypercalcemia. Endocrinology. 1986 Nov;119(5):2313–2324. doi: 10.1210/endo-119-5-2313. [DOI] [PubMed] [Google Scholar]
- Holzer-Petsche U., Lembeck F. Systemic capsaicin treatment impairs the micturition reflex in the rat. Br J Pharmacol. 1984 Dec;83(4):935–941. doi: 10.1111/j.1476-5381.1984.tb16534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägele E. O., Schaich E., Rauscher E., Lehmann P., Bürk H., Wahlefeld A. W. Mechanism of action of human pancreatic and salivary alpha-amylase on alpha-4-nitrophenyl maltoheptaoside substrate. Clin Chem. 1982 Nov;28(11):2201–2205. [PubMed] [Google Scholar]
- Ju G., Hökfelt T., Brodin E., Fahrenkrug J., Fischer J. A., Frey P., Elde R. P., Brown J. C. Primary sensory neurons of the rat showing calcitonin gene-related peptide immunoreactivity and their relation to substance P-, somatostatin-, galanin-, vasoactive intestinal polypeptide- and cholecystokinin-immunoreactive ganglion cells. Cell Tissue Res. 1987 Feb;247(2):417–431. doi: 10.1007/BF00218323. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Starke K. Substanz P und Speichelsekretion. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;259(5):375–385. [PubMed] [Google Scholar]


