Abstract
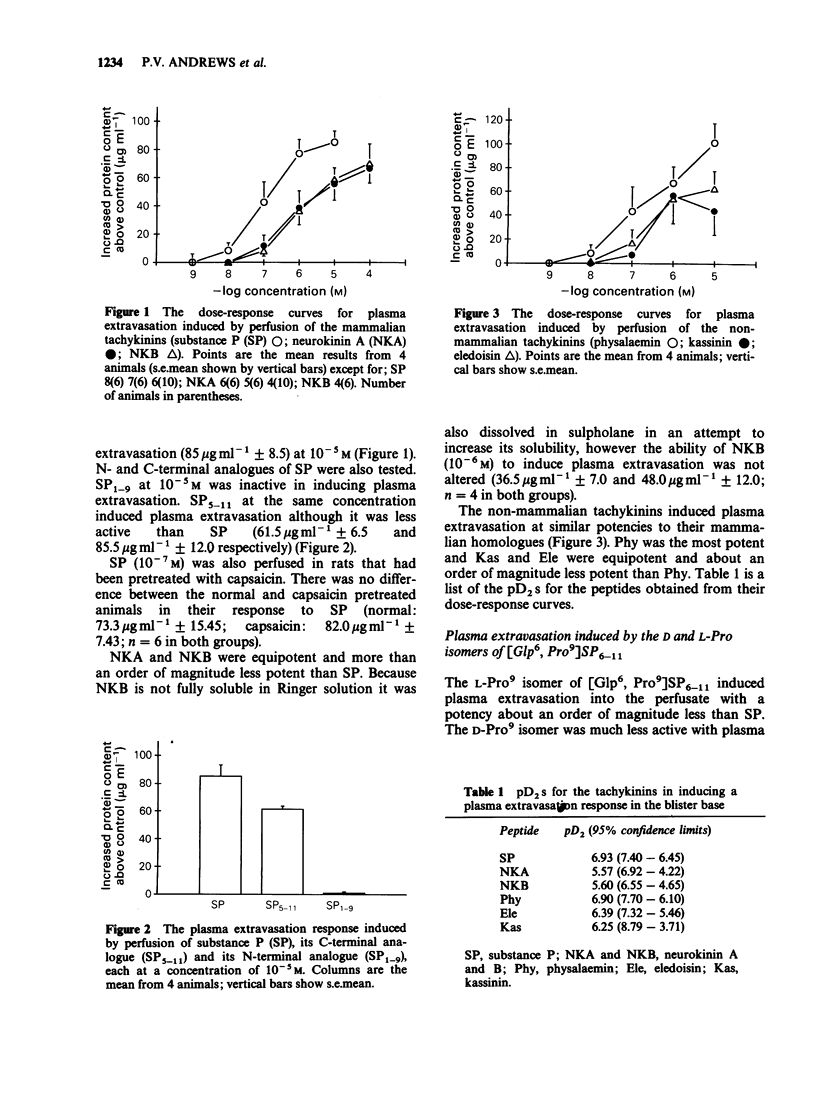
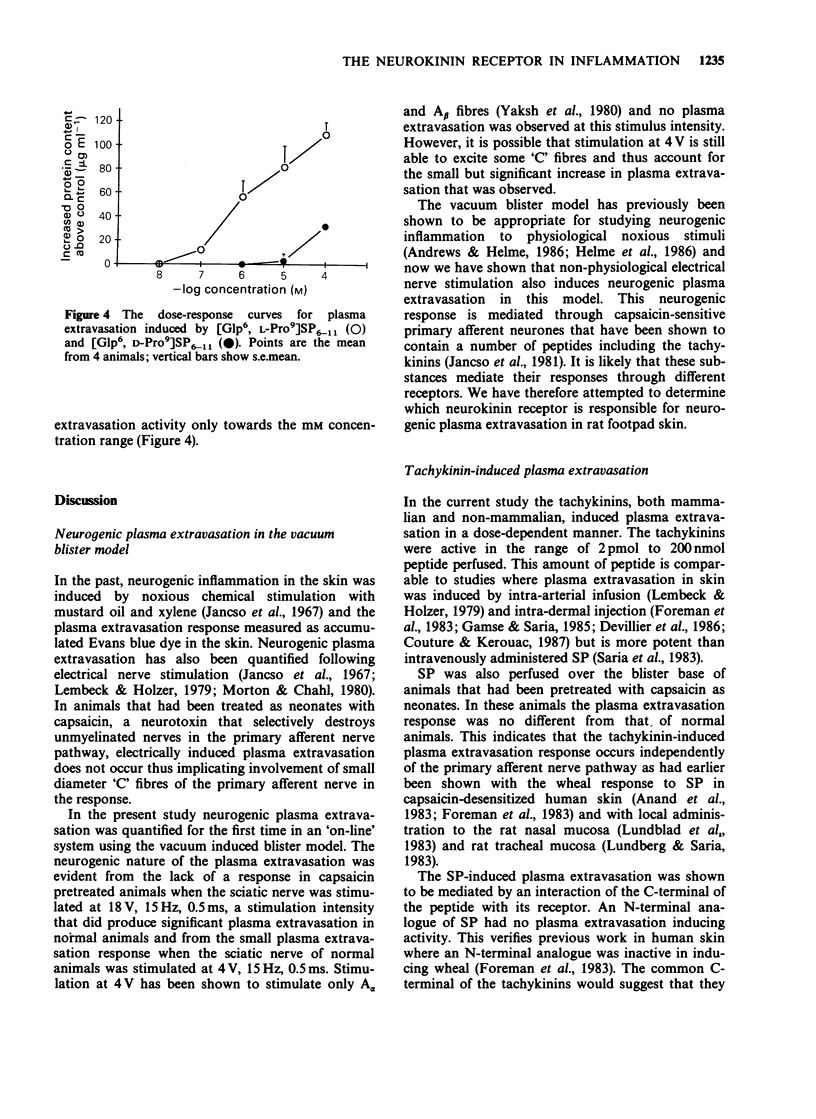
1. Plasma extravasation was induced by electrical nerve stimulation and by perfusion of tachykinins over a vacuum-induced blister base on rat footpad. 2. Stimulation of the sciatic nerve (18 V, 15 Hz, 0.5 ms) for 20 min produced a significant increase in the protein content of the perfusate. The response in capsaicin pretreated rats was only 4% of the control response. This indicates that the electrically-induced plasma extravasation response was mediated by capsaicin-sensitive sensory fibres. 3. Exogenous perfusion of the mammalian tachykinins substance P, neurokinin A and neurokinin B and the non-mammalian tachykinins physalaemin, kassinin and eledoisin was used to determine the tachykinin receptor type mediating the plasma extravasation response. Dose-response curves of the tachykinins (10(-9) M-10(-4) M) gave a rank order of potency of substance P = physalaemin greater than eledoisin greater than or equal to kassinin greater than neurokinin B = neurokinin A. 4. In addition, specific agonists of neurokinin receptors were perfused. Perfusion of [Glp6, D-Pro9] SP6-11 and [Glp6, L-Pro9]SP6-11 demonstrated that the L-Pro isomer was much more potent than the D-Pro isomer. 5. The rank order of potency and the greater potency of [Glp6, L-Pro9]SP6-11 over its D-isomer indicate an NK-1 neurokinin receptor mediates plasma extravasation in rat footpad skin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand P., Bloom S. R., McGregor G. P. Topical capsaicin pretreatment inhibits axon reflex vasodilatation caused by somatostatin and vasoactive intestinal polypeptide in human skin. Br J Pharmacol. 1983 Apr;78(4):665–669. doi: 10.1111/j.1476-5381.1983.tb09418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. V., Helme R. D. Neurogenic plasma extravasation in response to mechanical, chemical and thermal stimuli. Clin Exp Neurol. 1987;23:95–100. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bristow D. R., Curtis N. R., Suman-Chauhan N., Watling K. J., Williams B. J. Effects of tachykinins on inositol phospholipid hydrolysis in slices of hamster urinary bladder. Br J Pharmacol. 1987 Jan;90(1):211–217. doi: 10.1111/j.1476-5381.1987.tb16842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck S. H., Burcher E., Shults C. W., Lovenberg W., O'Donohue T. L. Novel pharmacology of substance K-binding sites: a third type of tachykinin receptor. Science. 1984 Nov 23;226(4677):987–989. doi: 10.1126/science.6095447. [DOI] [PubMed] [Google Scholar]
- Couture R., Kérouac R. Plasma protein extravasation induced by mammalian tachykinins in rat skin: influence of anaesthetic agents and an acetylcholine antagonist. Br J Pharmacol. 1987 Jun;91(2):265–273. doi: 10.1111/j.1476-5381.1987.tb10281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Orléans-Juste P., Dion S., Drapeau G., Regoli D. Different receptors are involved in the endothelium-mediated relaxation and the smooth muscle contraction of the rabbit pulmonary artery in response to substance P and related neurokinins. Eur J Pharmacol. 1986 Jun 5;125(1):37–44. doi: 10.1016/0014-2999(86)90081-6. [DOI] [PubMed] [Google Scholar]
- Devillier P., Regoli D., Asseraf A., Descours B., Marsac J., Renoux M. Histamine release and local responses of rat and human skin to substance P and other mammalian tachykinins. Pharmacology. 1986;32(6):340–347. doi: 10.1159/000138190. [DOI] [PubMed] [Google Scholar]
- Devillier P., Renoux M., Giroud J. P., Regoli D. Peptides and histamine release from rat peritoneal mast cells. Eur J Pharmacol. 1985 Oct 29;117(1):89–96. doi: 10.1016/0014-2999(85)90475-3. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Jordan C. C., Oehme P., Renner H. Structure-activity relationships for some substance P-related peptides that cause wheal and flare reactions in human skin. J Physiol. 1983 Feb;335:449–465. doi: 10.1113/jphysiol.1983.sp014543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamse R., Saria A. Potentiation of tachykinin-induced plasma protein extravasation by calcitonin gene-related peptide. Eur J Pharmacol. 1985 Aug 7;114(1):61–66. doi: 10.1016/0014-2999(85)90520-5. [DOI] [PubMed] [Google Scholar]
- Helme R. D., Andrews P. V., Watson B. A. Neurogenic inflammation caused by wool fabric in the rat; possible mediation by substance P. Neurosci Lett. 1986 May 23;66(3):333–337. doi: 10.1016/0304-3940(86)90041-8. [DOI] [PubMed] [Google Scholar]
- Helme R. D., White D. M., Andrews P. V. Neurogenic inflammation in skin blisters. Exp Brain Res. 1985;59(2):382–387. doi: 10.1007/BF00230918. [DOI] [PubMed] [Google Scholar]
- Jancsó G., Hökfelt T., Lundberg J. M., Kiraly E., Halász N., Nilsson G., Terenius L., Rehfeld J., Steinbusch H., Verhofstad A. Immunohistochemical studies on the effect of capsaicin on spinal and medullary peptide and monoamine neurons using antisera to substance P, gastrin/CCK, somatostatin, VIP, enkephalin, neurotensin and 5-hydroxytryptamine. J Neurocytol. 1981 Dec;10(6):963–980. doi: 10.1007/BF01258524. [DOI] [PubMed] [Google Scholar]
- Jancsó G., Kiraly E., Jancsó-Gábor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977 Dec 22;270(5639):741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967 Sep;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer R., Wormser U., Friedman Z. Y., Gilon C., Chorev M., Selinger Z. Neurokinin B is a preferred agonist for a neuronal substance P receptor and its action is antagonized by enkephalin. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7444–7448. doi: 10.1073/pnas.82.21.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. M., Campbell N. J., Williams B. J., Iversen L. L. Multiple tachykinin binding sites in peripheral tissues and in brain. Eur J Pharmacol. 1986 Nov 4;130(3):209–217. doi: 10.1016/0014-2999(86)90270-0. [DOI] [PubMed] [Google Scholar]
- Lee C. M., Iversen L. L., Hanley M. R., Sandberg B. E. The possible existence of multiple receptors for substance P. Naunyn Schmiedebergs Arch Pharmacol. 1982 Mar;318(4):281–287. doi: 10.1007/BF00501166. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Capsaicin-induced desensitization of airway mucosa to cigarette smoke, mechanical and chemical irritants. Nature. 1983 Mar 17;302(5905):251–253. doi: 10.1038/302251a0. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Santicioli P., Giuliani S., Regoli D., Meli A. Activation of micturition reflex by substance P and substance K: indirect evidence for the existence of multiple tachykinin receptors in the rat urinary bladder. J Pharmacol Exp Ther. 1986 Jul;238(1):259–266. [PubMed] [Google Scholar]
- Mizrahi J., Dion S., D'Orléans-Juste P., Escher E., Drapeau G., Regoli D. Tachykinin receptors in smooth muscles: a study with agonists (substance P, neurokinin A) and antagonists. Eur J Pharmacol. 1985 Nov 26;118(1-2):25–36. doi: 10.1016/0014-2999(85)90659-4. [DOI] [PubMed] [Google Scholar]
- Morton C. R., Chahl L. A. Pharmacology of the neurogenic oedema response to electrical stimulation of the saphenous nerve in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1980 Nov;314(3):271–276. doi: 10.1007/BF00498549. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Kanazawa I., Kimura S. Regional distribution of substance P, neurokinin alpha and neurokinin beta in rat spinal cord, nerve roots and dorsal root ganglia, and the effects of dorsal root section or spinal transection. Brain Res. 1985 Dec 16;359(1-2):152–157. doi: 10.1016/0006-8993(85)91423-4. [DOI] [PubMed] [Google Scholar]
- Regoli D., Escher E., Drapeau G., D'Orléans-Juste P., Mizrahi J. Receptors for substance P. III. Classification by competitive antagonists. Eur J Pharmacol. 1984 Jan 27;97(3-4):179–189. doi: 10.1016/0014-2999(84)90449-7. [DOI] [PubMed] [Google Scholar]
- Saria A., Lundberg J. M., Skofitsch G., Lembeck F. Vascular protein linkage in various tissue induced by substance P, capsaicin, bradykinin, serotonin, histamine and by antigen challenge. Naunyn Schmiedebergs Arch Pharmacol. 1983 Nov;324(3):212–218. doi: 10.1007/BF00503897. [DOI] [PubMed] [Google Scholar]
- White D. M., Helme R. D. Release of substance P from peripheral nerve terminals following electrical stimulation of the sciatic nerve. Brain Res. 1985 Jun 10;336(1):27–31. doi: 10.1016/0006-8993(85)90412-3. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Jessell T. M., Gamse R., Mudge A. W., Leeman S. E. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980 Jul 10;286(5769):155–157. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]


