Abstract
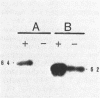
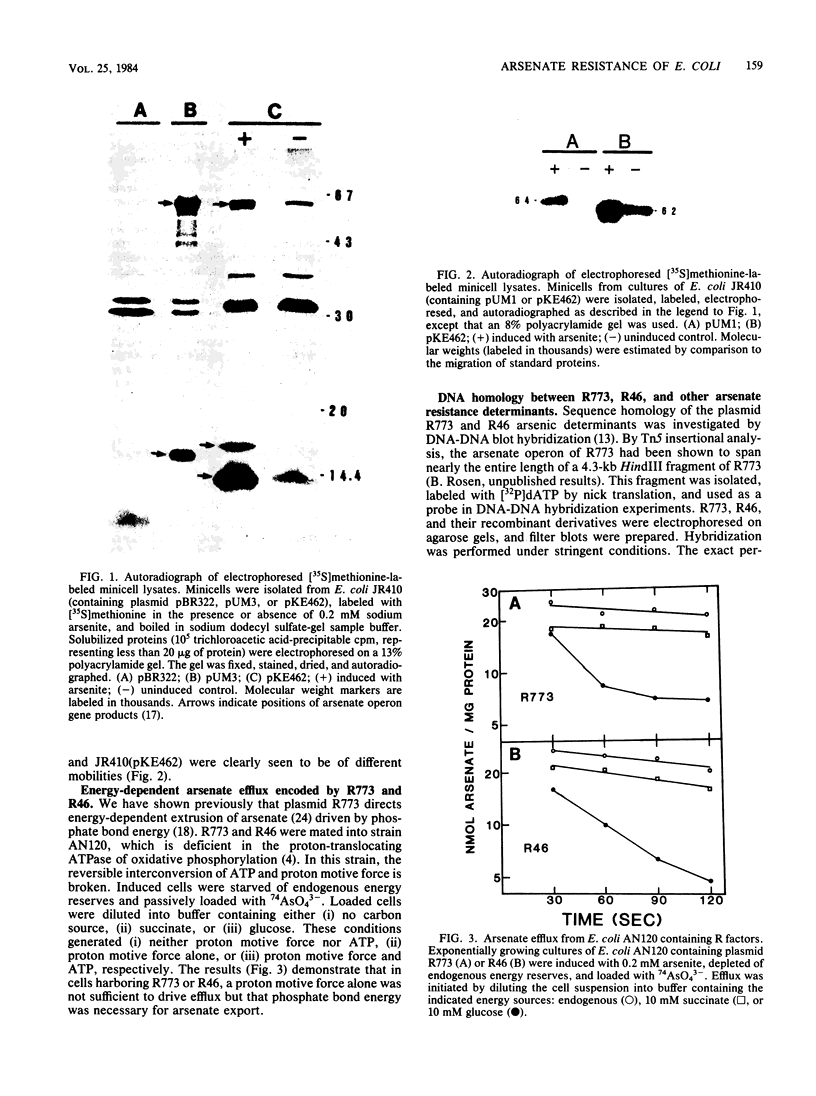
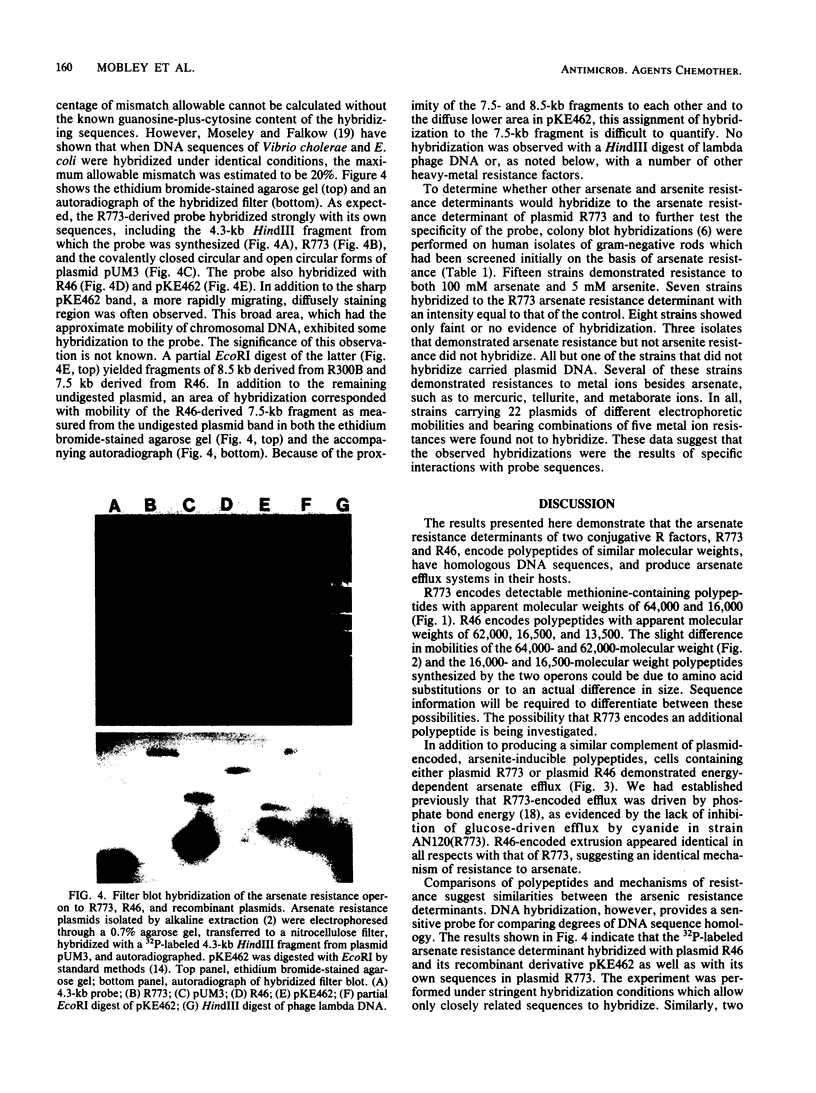
Escherichia coli bearing R factors R773 or R46 or hybrid recombinant plasmids carrying the arsenic resistance determinants derived from these plasmids synthesized inducible polypeptides of similar apparent molecular weights when exposed to arsenite salts (R773 derivative, 64,000 and 16,000; R46 derivative, 62,000, 16,500, and 13,500). In addition, both plasmids encoded energy-dependent arsenate efflux systems and demonstrated DNA sequence homology by filter blot hybridization. Human isolates of arsenate- and arsenite-resistant enterobacteria were tested for homology with the arsenate operon of R773 by colony blot hybridization. Approximately one-third of the isolates hybridized strongly, and two-thirds showed little or no evidence of homology, suggesting the presence of two or more genetically distinct arsenate resistant determinants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Willetts N. S. A physical and genetic map of the IncN plasmid R46. Plasmid. 1981 Mar;5(2):188–201. doi: 10.1016/0147-619x(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz F., Zabielski J., Philipson L., Lindberg M. DNA homology between the arsenate resistance plasmid pSX267 from Staphylococcus xylosus and the penicillinase plasmid pI258 from Staphylococcus aureus. Plasmid. 1983 Mar;9(2):126–137. doi: 10.1016/0147-619x(83)90015-x. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Baumberg S. Resistance to arsenic compounds conferred by a plasmid transmissible between strains of Escherichia coli. J Bacteriol. 1973 Jul;115(1):459–460. doi: 10.1128/jb.115.1.459-460.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes V. M., Datta N. Conjugative plasmids in bacteria of the 'pre-antibiotic' era. Nature. 1983 Apr 21;302(5910):725–726. doi: 10.1038/302725a0. [DOI] [PubMed] [Google Scholar]
- Jackson W. J., Summers A. O. Polypeptides encoded by the mer operon. J Bacteriol. 1982 Feb;149(2):479–487. doi: 10.1128/jb.149.2.479-487.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez B., Tachibana C., Levy S. B. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980 Mar;3(2):99–108. doi: 10.1016/0147-619x(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Chen C. M., Silver S., Rosen B. P. Cloning and expression of R-factor mediated arsenate resistance in Escherichia coli. Mol Gen Genet. 1983;191(3):421–426. doi: 10.1007/BF00425757. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Rosen B. P. Energetics of plasmid-mediated arsenate resistance in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6119–6122. doi: 10.1073/pnas.79.20.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley S. L., Falkow S. Nucleotide sequence homology between the heat-labile enterotoxin gene of Escherichia coli and Vibrio cholerae deoxyribonucleic acid. J Bacteriol. 1980 Oct;144(1):444–446. doi: 10.1128/jb.144.1.444-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter F. D., Silver S., Ong C., Nakahara H. Selection for mercurial resistance in hospital settings. Antimicrob Agents Chemother. 1982 Nov;22(5):852–858. doi: 10.1128/aac.22.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C. E., McNeill W. F., Farrar W. E., Jr Evolution of multiple-antibiotic-resistance plasmids mediated by transposable plasmid deoxyribonucleic acid sequences. J Bacteriol. 1979 Nov;140(2):713–719. doi: 10.1128/jb.140.2.713-719.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Budd K., Leahy K. M., Shaw W. V., Hammond D., Novick R. P., Willsky G. R., Malamy M. H., Rosenberg H. Inducible plasmid-determined resistance to arsenate, arsenite, and antimony (III) in escherichia coli and Staphylococcus aureus. J Bacteriol. 1981 Jun;146(3):983–996. doi: 10.1128/jb.146.3.983-996.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Keach D. Energy-dependent arsenate efflux: the mechanism of plasmid-mediated resistance. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6114–6118. doi: 10.1073/pnas.79.20.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W. Arsenic resistance in enterobacteria: its transmission by conjugation and by phage. J Gen Microbiol. 1978 Nov;109(1):49–56. doi: 10.1099/00221287-109-1-49. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Jacoby G. A. Plasmid-determined resistance to tellurium compounds. J Bacteriol. 1977 Jan;129(1):276–281. doi: 10.1128/jb.129.1.276-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]