Abstract
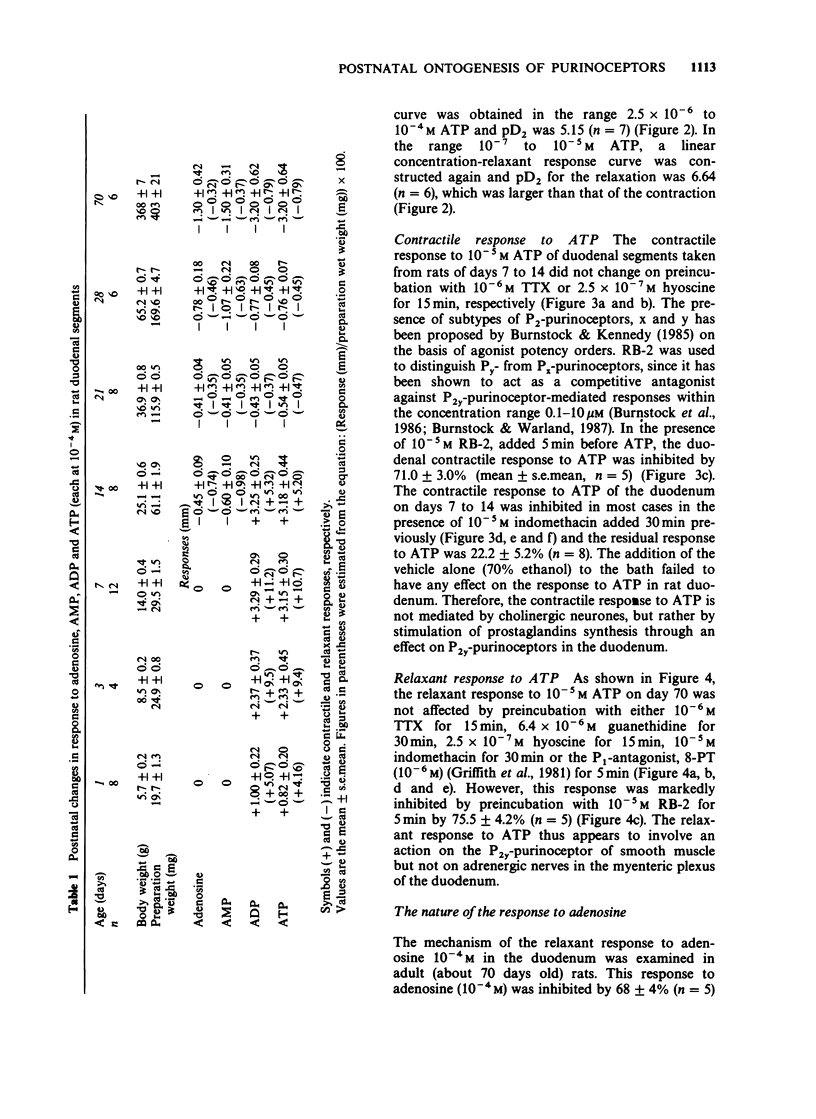
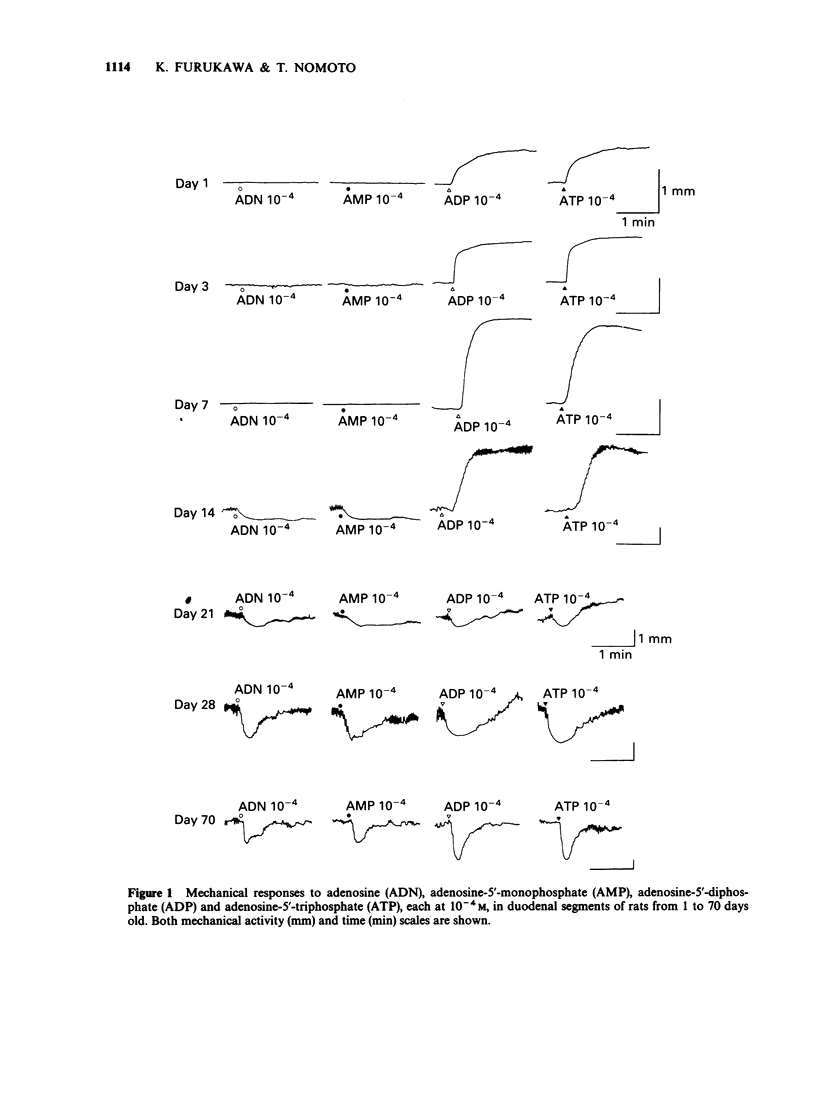
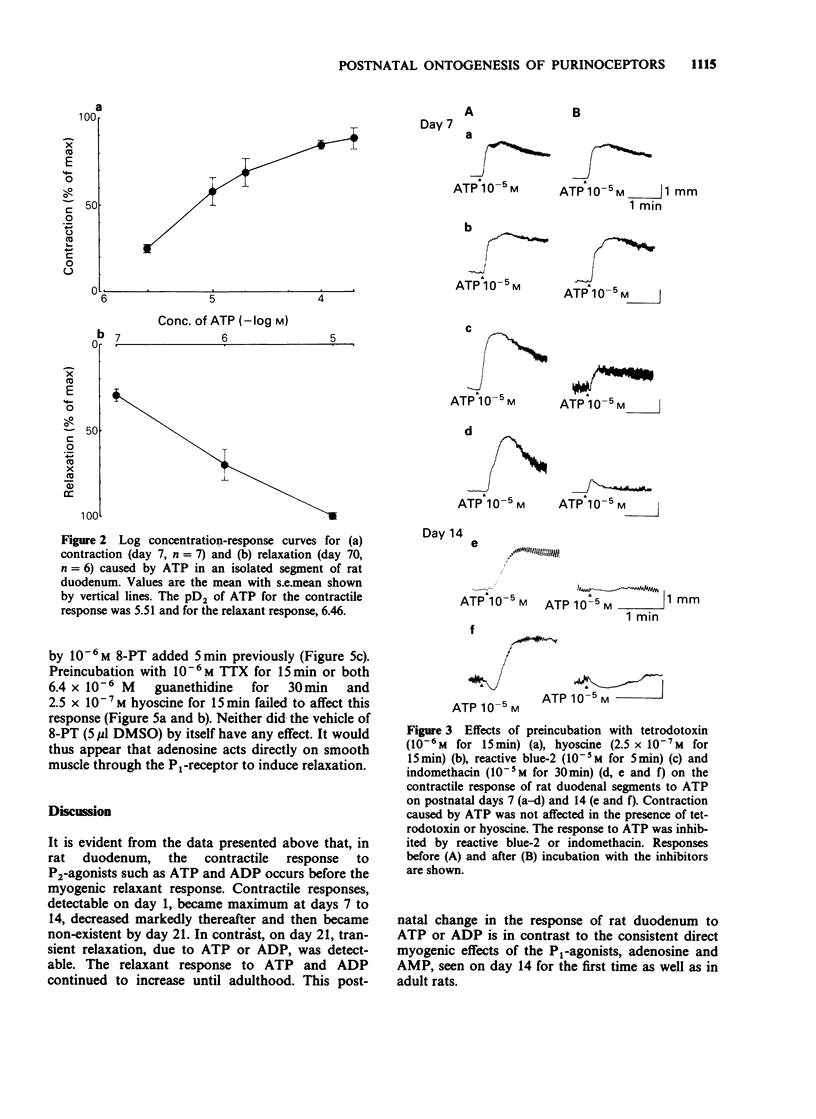
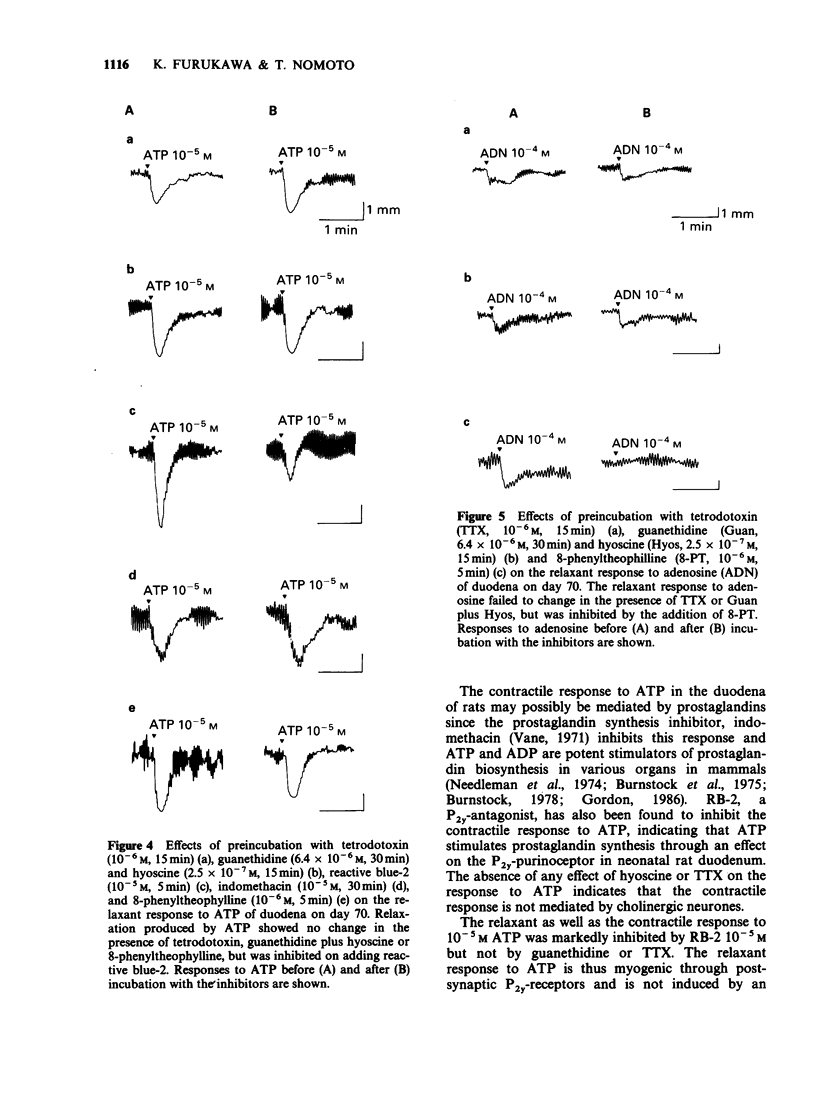
1. The effects of adenosine and adenine nucleotides were studied in rat duodenum from postnatal day 1 to day 70. The mechanical activity of duodenal segments was recorded through an isotonic transducer connected to a polygraphic recorder. 2. In rat duodenal segments, adenosine-5'-triphosphate (ATP, 10(-4) M) and adenosine-5'-diphosphate (ADP, 10(-4) M) produced a contractile response on postnatal day 1. This response increased with age, peaking on day 7, followed by a gradual decrease and was non-existent by day 21. In contrast, a relaxant response to ATP and ADP was apparent on day 21, and continued to increase up to day 70. 3. The contraction caused by ATP was inhibited by indomethacin or the P2y-purinoceptor antagonist, reactive blue-2 but not by tetrodotoxin or hyoscine. Thus, it may be mediated by production of prostaglandin through the P2y-purinoceptor. The relaxation produced by ATP was inhibited by reactive blue-2 but not by tetrodotoxin, guanethidine or the P1-purinoceptor antagonist, 8-phenyltheophylline indicating that ATP acts on smooth muscle directly through the P2y-purinoceptor. The pD2 for the contractile response to ATP was 5.15 on day 7 and that for the relaxant response, 6.64 on day 70. 4. Adenosine (10(-4) M) and adenosine-5'-monophosphate (AMP, 10(-4) M) elicited no response before day 14. On day 14, both adenosine and AMP produced a small relaxant response which increased with age. The relaxation produced by adenosine was inhibited by 8-phenyltheophylline but not by tetrodotoxin or guanethidine, indicating that it is mediated by an action on the P1-purinoceptor of smooth muscle.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A., Eley K. G., Scholes G. B. Effect of prostaglandins E1 and E2 on intestinal motility in the guinea-pig and rat. Br J Pharmacol. 1968 Nov;34(3):639–647. doi: 10.1111/j.1476-5381.1968.tb08493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Burnstock G. Evidence in support of the P1/P2 purinoceptor hypothesis in the guinea-pig taenia coli. Br J Pharmacol. 1981 Jul;73(3):617–624. doi: 10.1111/j.1476-5381.1981.tb16796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Paddle B., Staszewska-Barczak J. Evidence that prostaglandin is responsible for the 'rebound contraction' following stimulation of non-adrenergic, non-cholinergic ('purinergic') inhibitory nerves. Eur J Pharmacol. 1975 Apr;31(2):360–362. doi: 10.1016/0014-2999(75)90060-6. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Burnstock G., Warland J. J. P2-purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2y-but not the P2x-purinoceptor. Br J Pharmacol. 1987 Feb;90(2):383–391. doi: 10.1111/j.1476-5381.1987.tb08968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks T., Burnstock G. Effects of neuronal polypeptides on intestinal smooth muscle; a comparison with non-adrenergic, non-cholinergic nerve stimulation and ATP. Eur J Pharmacol. 1979 Mar 1;54(3):251–259. doi: 10.1016/0014-2999(79)90084-0. [DOI] [PubMed] [Google Scholar]
- Ferrero J. D., Frischknecht R. Different effector mechanisms for ATP and adenosine hyperpolarization in the guinea-pig taenia coli. Eur J Pharmacol. 1983 Jan 28;87(1):151–154. doi: 10.1016/0014-2999(83)90063-8. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Tonoue T., Nomoto T. Postnatal change in receptivity for methionine5-enkephalin in rat duodenum: transition from neurogenic to myogenic receptivity. Eur J Pharmacol. 1982 Aug 27;82(3-4):161–166. doi: 10.1016/0014-2999(82)90505-2. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith S. G., Meghji P., Moody C. J., Burnstock G. 8-phenyltheophylline: a potent P1-purinoceptor antagonist. Eur J Pharmacol. 1981 Oct 15;75(1):61–64. doi: 10.1016/0014-2999(81)90346-0. [DOI] [PubMed] [Google Scholar]
- Henning S. J. Postnatal development: coordination of feeding, digestion, and metabolism. Am J Physiol. 1981 Sep;241(3):G199–G214. doi: 10.1152/ajpgi.1981.241.3.G199. [DOI] [PubMed] [Google Scholar]
- Manzini S., Maggi C. A., Meli A. Further evidence for involvement of adenosine-5'-triphosphate in non-adrenergic non-cholinergic relaxation of the isolated rat duodenum. Eur J Pharmacol. 1985 Jul 31;113(3):399–408. doi: 10.1016/0014-2999(85)90088-3. [DOI] [PubMed] [Google Scholar]
- Manzini S., Maggi C. A., Meli A. Pharmacological evidence that at least two different non-adrenergic non-cholinergic inhibitory systems are present in the rat small intestine. Eur J Pharmacol. 1986 Apr 16;123(2):229–236. doi: 10.1016/0014-2999(86)90664-3. [DOI] [PubMed] [Google Scholar]
- Needleman P., Minkes M. S., Douglas J. R., Jr Stimulation of prostaglandin biosynthesis by adenine nucleotides. Profile of prostaglandin release by perfused organs. Circ Res. 1974 Apr;34(4):455–460. doi: 10.1161/01.res.34.4.455. [DOI] [PubMed] [Google Scholar]
- Satchell D. G., Maguire M. H. Evidence for separate receptors for ATP and adenosine in the guinea-pig taenia coli. Eur J Pharmacol. 1982 Jul 30;81(4):669–672. doi: 10.1016/0014-2999(82)90358-2. [DOI] [PubMed] [Google Scholar]
- Thomson A. B., Keelan M. The development of the small intestine. Can J Physiol Pharmacol. 1986 Jan;64(1):13–29. doi: 10.1139/y86-003. [DOI] [PubMed] [Google Scholar]
- Tonoue T., Furukawa K., Nomoto T. Transition from neurogenic to myogenic receptivity for thyrotropin-releasing hormone (TRH) in the duodenum of the neonatal rat. Endocrinology. 1981 Feb;108(2):723–725. doi: 10.1210/endo-108-2-723. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]



